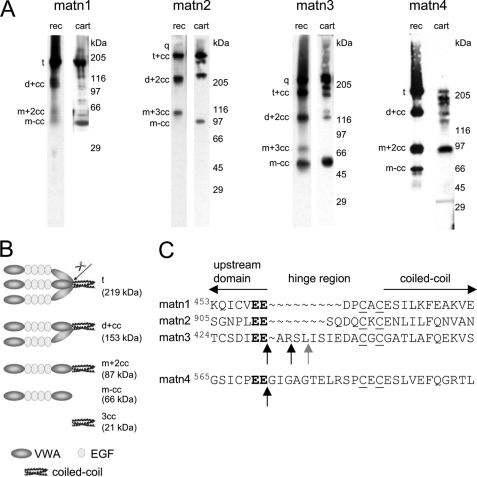
FIGURE 1.
Matrilin processing. A, proteins in conditioned cell culture media of 293EBNA cells transfected with matrilin-1, -2, -3, or -4 (rec) and extracts of murine articular cartilage (cart) were separated by non-reducing SDS-PAGE on 4–12% SDS-PAGE gels and transferred to nitrocellulose. Matrilins were subsequently detected with affinity purified antibodies specific for each matrilin or for the StrepII tag (matn2 rec). Note that the lane showing the recombinant matrilin-1 was overexposed to better visualize also the weak processed bands. For nomenclature, see Footnote 3 (5). B, schematic representation of uncleaved matrilin-4 trimers (t) and fragments resulting from proteolytic processing. Cleavage in the hinge region (arrow) connecting the second von Willebrand factor A-like (VWA) and the C-terminal coiled-coil oligomerization domain generates a mixture of processed fragments comprised of dimers (d+cc) and monomers (m+2cc) with fully assembled trimeric coiled-coils, completely processed trimeric coiled-coils (3cc), as well as the cleaved-off monomer (m-cc) lacking the coiled-coil region. C, amino acid sequence alignment of the murine matrilin hinge regions. The matrilin-4 cleavage motif (bold) is conserved in the protein family. Black vertical arrows mark cleavage sites identified by N-terminal Edman sequencing and MALDI-TOF mass spectrometry. The cleavage site marked by the gray vertical arrow was found only by MALDI-TOF. Horizontal arrows mark the adjacent N-terminal domains and the C-terminal coiled-coil domains, respectively. The cysteine residues involved in intermolecular disulfide bond formation are underlined.