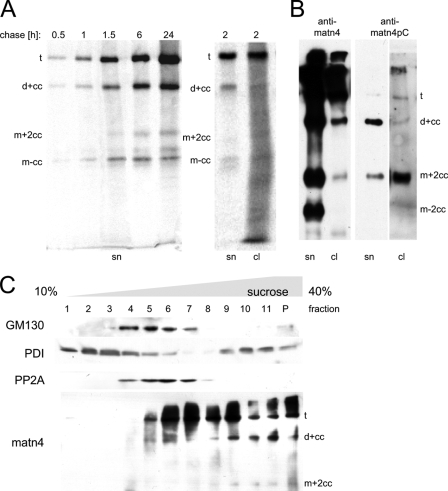
FIGURE 4.
Intracellular processing of recombinant matrilin-4. A, 293EBNA cells stably transfected with StrepII-tagged matrilin-4 were exposed to a pulse of 35S-labeled methionine and cysteine. Cell culture supernatants were harvested at the indicated time points (chase). For the 2-h time point both the supernatant (sn) and the cell lysate (cl) are shown. Radiolabeled matrilin-4 was precipitated using Streptactin-Sepharose beads. The precipitated proteins were separated by non-reducing SDS-PAGE, the gel was dried and radioactivity detected with a phosphorimager. B, immunoblot of recombinant matrilin-4 either from cell culture supernatants (sn) or cell lysates (cl) after removing potential cell surface matrilin-4 by trypsin treatment. The processing patterns were detected using antibodies against all matrilin-4 forms (anti-matn4) or exclusively against the N-terminal neo-epitope of the cleaved C-terminal coiled-coil fragment (anti-matn4pC), which is present in processed dimers and monomers with still trimeric coiled-coils (d+cc, m+2cc) (see Footnote 3). Note that the lane showing the cell lysate probed with the neo-epitope antibody was overexposed to better visualize the bands. C, cells expressing recombinant matrilin-4 were homogenized and subcellular fractions were separated by centrifugation in a 10–40% sucrose gradient, poured on a cushion of 40% sucrose. Fractions 1 to 11 and a pellet fraction (p) were applied to SDS-PAGE and analyzed in subsequent immunoblots with antibodies specific for the cis-Golgi marker GM130, the endoplasmic reticulum resident protein-disulfide isomerase (PDI), the cytoplasmic protein phosphatase 2A (PP2A), and matrilin-4 (matn4).