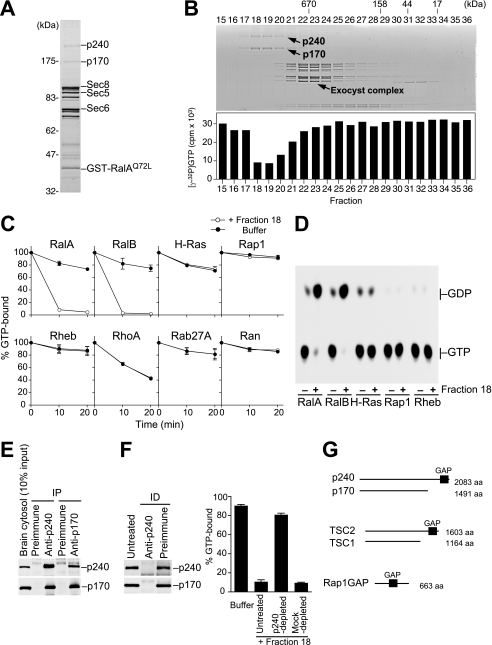
FIGURE 1.
The p240-p170 complex purified from porcine brain has RalGAP activity. A, RalAQ72L affinity column eluate was analyzed by SDS-PAGE and Coomassie Blue staining. Components of the exocyst complex (Sec8, Sec5, and Sec6) and GST-RalAQ72L that had leaked from the column are indicated, as identified by Western blotting (not shown). B, upper panel, the mixture of RalAQ72L-binding proteins was separated on a Superose 6 gel filtration column, and the resulting fractions were analyzed by SDS-PAGE and Coomassie blue staining. Lower panel, each fraction was tested for RalGAP activity by a filter binding assay using [γ-32P]GTP-loaded RalA as described under “Experimental Procedures.” C, various Ras family GTPases preloaded with [γ-32P]GTP were incubated at 30 °C for the indicated periods with (open circles) or without (closed circles) fraction 18, and the [γ-32P]GTP remaining bound to GTPases was measured by the filter binding assay (mean ± S.E., n = 2). D, Ras subfamily GTPases preloaded with [α-32P]GTP were incubated at 30 °C for 10 min with (+) or without (−) fraction 18, and bound nucleotides were analyzed by thin layer chromatography and autoradiography. E, co-immunoprecipitation of p240 and p170 from porcine brain cytosol. IP, immunoprecipitation. F, immunodepletion of p240 from fraction 18 results in a concomitant depletion of p170 (left panel) and abolishes the GAP activity for RalA (right graph) (mean ± S.E., n = 3). ID, immunodepletion. G, schematic comparison of the domain structure of the p240-p170 complex, the TSC2-TSC1 complex, and Rap1GAP. aa, amino acids.