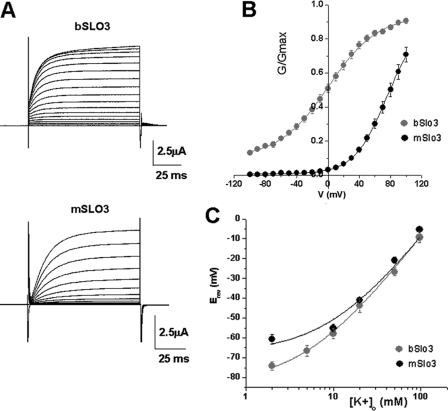
FIGURE 2.
bSLO3 channels activate at more negative potentials than mSLO3 channels and are less voltage-dependent. A, families of whole cell currents from bSLO3 and mSLO3 channels expressed in Xenopus oocytes evoked by voltage steps from −90 to +100 mV in 10-mV steps at Vh = −70 mV. B, G-V relationships for bSLO3 and mSLO3 currents. bSLO3 channels activate at more negative potentials, and the currents have a more shallow conductance-voltage relation than mSLO3. Note that there is significant bSLO3 conductance at −100 mV. The data were fitted with the Boltzmann equation (see “Materials and Methods”) with V½ = 0.49 ± 2.1 mV, k = 31.7 ± 2.2 (n = 21) for bSLO3; and V½ = 77.1.6 ± 4.9, k = 21.2 ± 1.01 for mSLO3 (n = 9). C, reversal potentials (Erev) plotted at different external K+ concentrations to illustrate the relative selectivity for K+ over Na+ for bSLO3 and mSLO3 channels. Reversal potentials were obtained by measuring tail currents at different [K+]o. [Na+]o was also varied so that the total concentration of monovalent cations was 98 mm. The data were fitted with the Goldman-Hodgkin-Katz equation, Erev = RT/F*ln([K+]o + P*([Na+]o))/([K+]i + P*[Na+]i), where P is the Na+/K+ permeability ratio and varied freely. For bSLO3 reversal potentials were obtained at 2, 5, 10, 20, 50, and 97 mm [K+]o (n = 12, 8, 6, 7, 9, and 4, respectively), and the calculated pNa+/pK+ was 0.05. For mSLO3 reversal potentials were obtained at 2, 10, 20, 50, and 97 mm [K+]o (n = 8, 8, 2, 6, and 4, respectively), which yielded a pNa+/pK+of 0.1. Fits were performed using Sigmaplot (Jandel). Another indication that bSLO3 had higher selectivity for K+ over Na+ was the fact that the resting potentials of eggs injected with bSLO3 were more negative, e.g. −63.6 ± 2.1 mV (n = 16) versus −34.4 ± 2.7 mV (n = 9) for eggs injected with mSLO3.