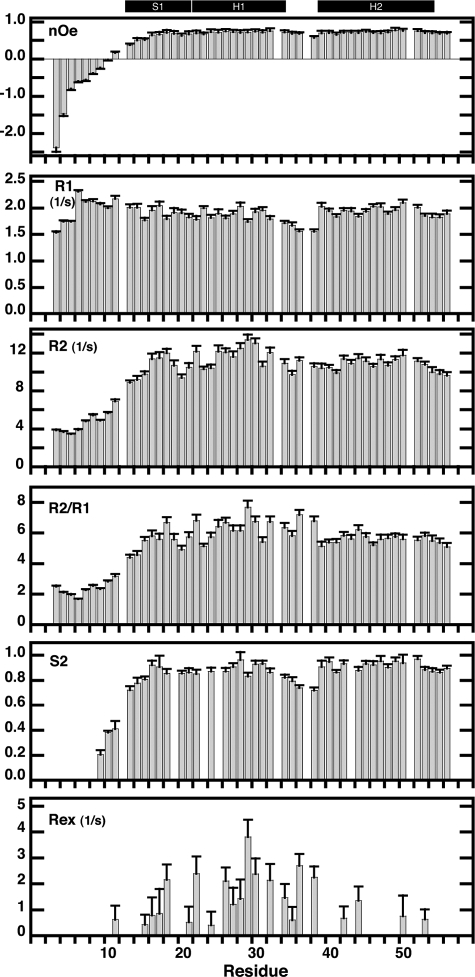
FIGURE 2.
15N relaxation rates (R2, R1 and R2/R1), heteronuclear 15N-1H NOE, and order (S2) and conformational exchange (Rex) Lipari-Szabo parameters of SvtR plotted as a function of residue number. Experiments were performed at 25 °C and a proton frequency of 499.8 MHz as described under “Experimental Procedures.” The secondary structure of SvtR is indicated on the top with black boxes (S = strand, H = helix). Relaxation data for residues 12, 33, 37, and 51 are not shown because of partial overlap of their corresponding signals. Data for residues 9 (overlap with 12) and 10 (overlap with 33) are included, however, because the amide group of these residues, which are located in the unfolded N-terminal region, gave strong signals that were less influenced by the overlapping signals. Order parameters are shown only for residues that were fit at a confidence level ≥ 0.9.