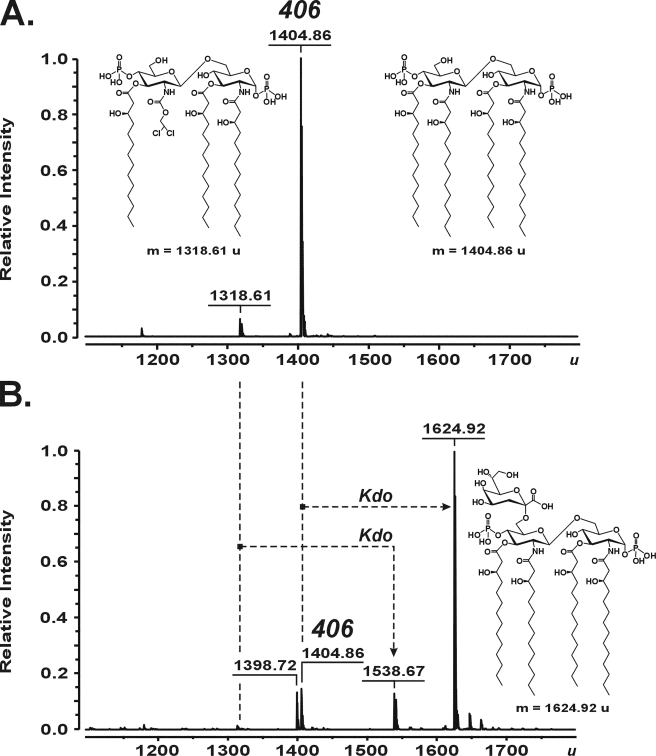
FIGURE 4.
Charge deconvoluted ESI FT-ICR mass spectra in negative ion mode, demonstrating the conversion of the tetraacyl-1,4′-bisphosphate lipid A precursor 406 (inset structure; 1404.86 u) (A) into Kdo-406 (inset structure; 1624.92 u) (B) by the Kdo transferase of A. aeolicus. The reaction mixtures containing 100 mm HEPES, pH 7.5, 10% glycerol, 10 mm MgCl2, 2 mm Kdo, 5 mm CTP, 3.2 mm Triton X-100, and 107 μm compound 406 were incubated at 60 °C for 1 h with no enzymes (A), and in the presence of 17 milliunits of purified KdsBAAE and 75 μm purified WaaAAAE (B). A triacylated byproduct of 406 synthesis (inset structure; 1318.61 u) (A) served as an acceptor for the WaaAAAE-catalyzed transfer of a single Kdo residue to yield the molecule at 1538.67 u (B). The peak at 1398.72 u (B) is probably a triacylated degradation product of Kdo-406 produced during sample preparation.