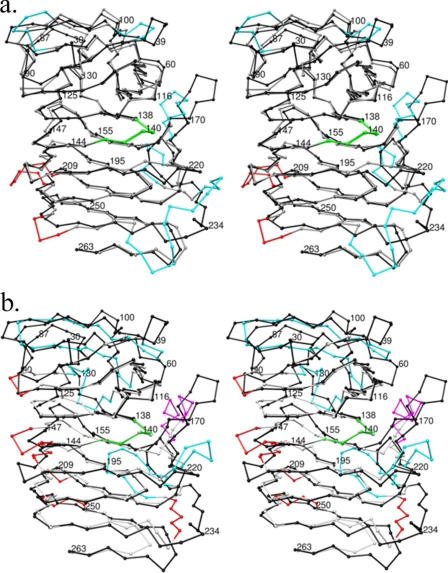
FIGURE 2.
The crystal structures of HpmA265, Fha30, and HMW1-PP show differences within the top and flanking anti-parallel β-sheet regions. a, the Cα coordinates have been superimposed between HpmA265 and Fha30. The trace for HpmA Cα atoms has been colored black in both images. Structural differences between HpmA265 and Fha30 have been colored cyan for the top and flanking regions apβ1, apβ2, and apβ3, while differences within β-arc regions have been colored red. The largest structural differences between HpmA265 and Fha30 lie at the flanking anti-parallel sheet apβ3. This shift accommodates a 12-residue insertion between β22 and β23 in HpmA265 and preserves the integrity of the β-helix core alignment. b, structural differences between HpmA265 and HMW1-PP have been colored cyan for anti-parallel regions, magenta for α-helix regions, and red for β-arcs. The large shift at the N-terminal end derives from a global superposition of three anti-parallel strands within HMW1-PP onto the four anti-parallel strands within HpmA265. This large difference stems from moving β2 of HMW1-PP between β1 and β2 of HpmA265. The N-terminal superposition also leaves β5 and β6 of HpmA265 without matching HMW1-PP strands. Additionally, HMW1-PP has an α-helix in place of two of the anti-parallel strands within apβ3. This creates another location of large structural difference. In both instances, the structural agreement between β-helix core Cα atoms is well maintained, especially within the NPNG motif (colored green).