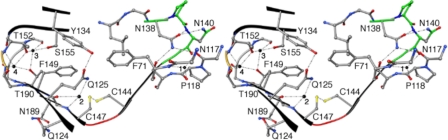
FIGURE 3.
Stabilization of the β-helix third coil. The stereo-diagram illustrates a view down the β-helical axis where partial coils above and below the CXXC motif have been maintained. Each β-arc connecting each of the three sides (a–c) has been colored independently. The β-arc containing the NPNG motif has been colored green, the β-arc between the C144:C147 disulfide bond has been colored red, and the third β-arc has been colored gold. Solvent molecules (1–4) have been represented as black spheres. Atoms have been colored by type: carbon (gray), oxygen (red), nitrogen (blue), and sulfur (yellow). The third coil utilizes a highly conserved hemagglutinin pattern, a CXXC motif, four buried solvent molecules (1–4), and a carboxyamide ladder and thereby, provides unique stabilization architecture within the three respective β-arcs. The first arc implements Asn-38 and Asn-40 from the conserved hemagglutinin pattern (NPNG) and solvent molecule (1) for stabilization. The series of hydrogen bonds within this arc forms a stabilizing bridge between coils 1, 2, and 3. The disulfide bond shared between Cys-144 and Cys-147 within the CXXC motif stabilizes the second β-arc. Additionally, solvent 2 forms a trifurcated hydrogen bond with Gly-148-CO, Gly-148-N, and Gln-125-Nϵ2. Hydrogen bonding between solvents 3 and 4 and neighboring protein atoms stabilize the single residue β-arc three and interconnect coils 3 and 4. Additionally, the carboxyamide ladder shared between Gln-124 of β9 (coil 2) and Asn-89 of β17 (coil 4) adds further stability to β12 (coil 3).