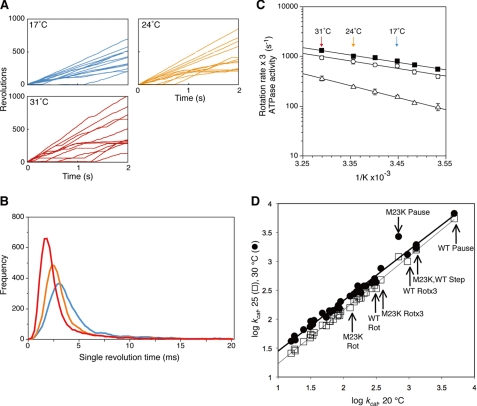
FIGURE 1.
Effect of temperature on the γ subunit rotation in F1. A, time courses of 60-nm gold bead rotation attached to the γ subunit were followed for 2 s at the indicated temperatures. B, single revolution times (time required for full 360° rotations) were obtained from ∼10 gold beads rotating at 17 °C (blue), 24 °C (orange), or 31 °C (red). C, Arrhenius analysis of rotation rates, obtained from the reciprocals of the geometric means of single revolution times (closed squares) or from arithmetic average of the number of rotations in 2 s (open circles). The rotation rates are reported based on the assumption that three ATP are hydrolyzed per 360° rotation, equivalent to ATPase activities (three times the number of rotations). Temperature dependence of bulk phase steady state ATPase activities is also shown (open triangles, the F1 used was the same as the rotation experiments but without the gold bead attached). The error bars show the standard error. D, linear free energy relationship of the ATP synthase. Linear free energy relationships between activities at 20 °C on the x axis compared with 25 °C (open squares) or 30 °C (closed circles) on the y axis. ATPase and ATP synthesis data are taken from a wide variety of sources as reported elsewhere (27, 39). The lines are linear regressions for each data set: the goodness of fit values, R2, for the 20–25 °C and the 20–30 °C data sets are 0.997 and 0.991, respectively. The arrows indicate the new data for wild-type (WT) and γM23K F1 rotations rates with or without multiplying by three (WT or M23K Rot or WT or M23K Rotx3, respectively), speed of the 120° step (M23K, WT Step), or pause durations (WT or M23K Pause).