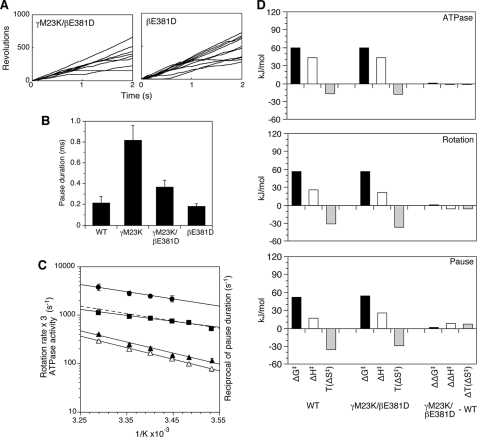
FIGURE 5.
Effect of temperature on γM23K/βE381D rotation. A, time courses of 60-nm gold bead rotation at 24 °C attached to the γ subunit of the γM23K/βE381D F1 (left) or βE381D F1 (right). B, average length of the pause duration of mutant F1 at 24 °C. The error bars indicate standard errors. C, Arrhenius analysis of the rotation data for γM23K/βE381D F1; the reciprocal of the pause duration (closed circles) and the rotation rates (closed squares) obtained from the reciprocals of the geometric means of single revolution times. The linear regression line from the rotation rates of wild-type F1 from Fig. 1C is shown for comparison (dotted line). The rotation rates are reported, as in Fig. 1, based on the assumption that three ATP are hydrolyzed per 360° rotation, equivalent to ATPase activities (three times the number of rotations). Temperature dependence of bulk phase steady state ATPase activities of the γM23K/βE381D F1 (open triangles) and βE381D F1 (closed triangles) are also shown. The error bars show the standard error. D, thermodynamic parameters of the γM23K/βE381D F1 compared with wild-type F1 calculated at 31 °C from Arrhenius data for bulk phase steady state ATPase activity, rotation rate, and the pause duration.