Figure 1.
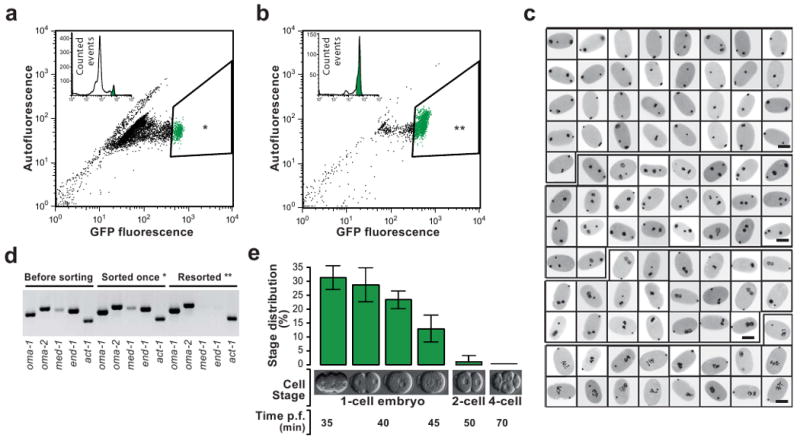
eFACS yields tens of thousands of cleanly staged one-cell embryos with at least 98% purity.
(a) A mixed population of embryos from the OMA-1::GFP strain is passed through a Fluorescent Activated Cell Sorter (FACS). Shown is the GFP fluorescent signal vs autofluorescence. One-cell embryos with a high GFP signal (3-5% of the initial population) are selected (*). 10,000 embryos are depicted in the scatter plot. (b) The first sort enriches the one-cell stage embryo population to ∼70%. 2,000 embryos are depicted in the scatter plot. Sorting once more (resorting) the same population (**) yields one-cell embryos at a purity of >98% (see below). (c) Developmental stage analysis of resorted embryos of a randomly selected sample (96 embryos). DAPI stained pronuclei appear black. All embryos are one-cell stage. Scale bars, 25μm. (d) RT-PCR analysis of once-sorted (*), Resorted embryos (**), and a mixed embryo population prior to sorting. In resorted embryos the one-cell stage specific marker genes oma-1 and oma-2 but not early zygotic (four-to-eight-cell stage) genes med-1 and end-1 are amplified. However, in embryos that were sorted only once, expression of med-1 and end-1 is still detectable. Act-1 was used as RT-PCR control. (e) Summary statistics of microscopic analyses of resorted embryos. The majority of embryos are in the pseudocleavage and chromosome condensation phase (30%) and in the pronuclear migration phase (30%) of the first cell cycle. Two-cell and older embryos represent less than 2% of the resorted embryo population (Error bars, s.d., n = 5, 100 embryos counted per replicate).
