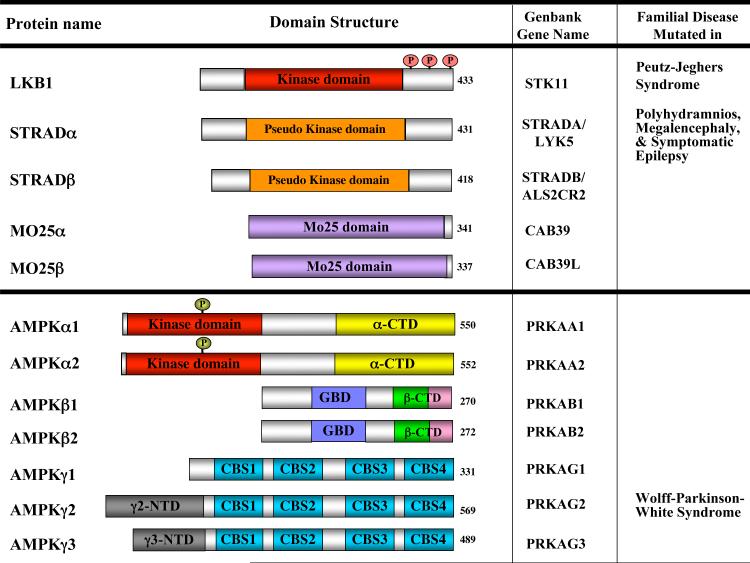
Figure 1. Schematic of the proteins in the LKB1 and AMPK kinase complexes.
Both LKB1 and AMPK exist in heterotrimeric protein complexes. Inactivating mutations in LKB1 underlie the inherited cancer disorder Peutz-Jeghers Syndrome. Most mutations affect the function of the kinase domain, indicating that the tumor suppressor function of LKB1 requires its kinase activity. In addition to deletions or frameshifts, several missense mutations have been found and most cluster to the kinase domain resulting in loss of kinase activity. A handful of mutations lie outside the kinase domain and some of these have been shown to result in decreased kinase activity due to disruption of protein-protein interactions between LKB1 and its regulatory subunits STRAD (STE20-related adapter protein) and Mo25, which appear to be necessary for its kinase activity186. Together, the genetic evidence indicate that the tumor suppressor function of LKB1 requires its kinase activity. While there is a single LKB1 gene in mammals, two STRAD and two Mo25 family members exist and mutations in STRADα underlie the development of an inherited epileptic disorder187. There are two known splice forms of LKB1 differing in the very C-terminal amino acids188, 189, and evidence suggests STRAD proteins undergo extensive alternative splicing as well190. Like LKB1, AMPK is composed of a catalytic subunit (α) and two regulatory subunits. The beta subunits contain a conserved glycogen binding domain which also modulates AMPK activity191. The gamma subunits contain a series of tandem repeats of crystathionine-β-synthase (CBS) domains to which molecules of AMP directly bind as revealed in recent X-ray crystallography studies192. Binding of AMP to AMPKγ is thought to promote phosphorylation of the critical activation loop threonine (Thr172) in AMPKα, which is required for AMPK activity, largely through suppression of phosphatase activity towards Thr172193. Mutation of some of these AMP-binding pockets in the AMPKγ2 gene lead to hypertrophic cardiomyopathy that is associated with Wolff-Parkinson-White syndrome194.