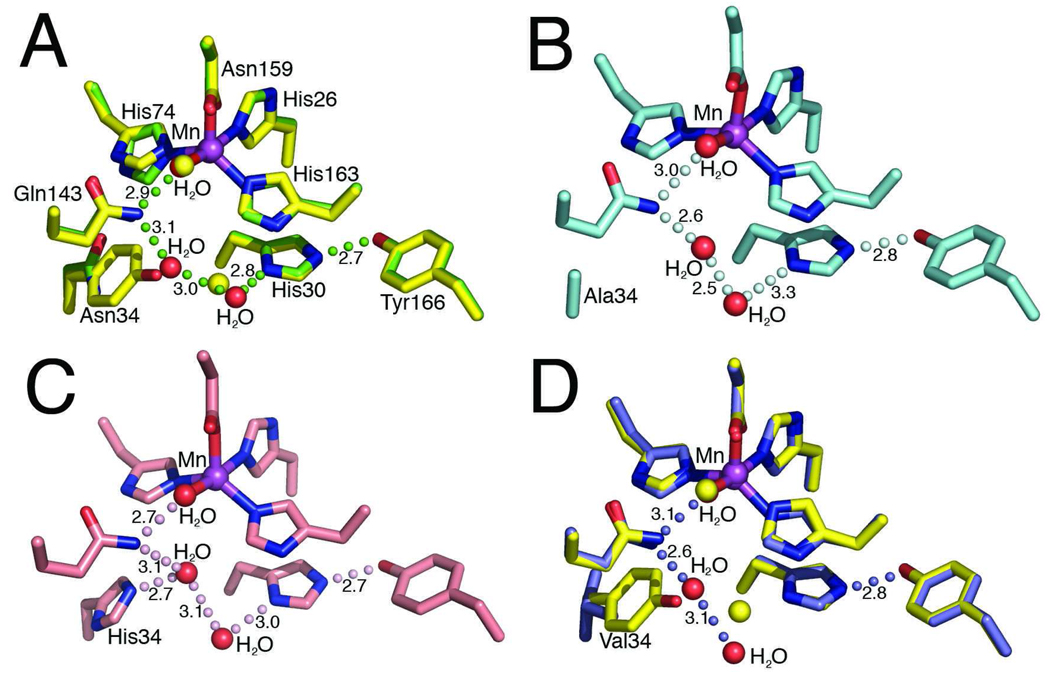
Fig 1.
MnSOD active site structure and residue 34 mutations. (A) Superimposition of the active site side chains, manganese ion, and ordered solvent molecules of the human wild-type MnSOD (yellow) and Y34N (green). Y34N waters are depicted in red (wild-type waters in yellow), green spheres depict the Y34N hydrogen bond relay and the bond distances, in Å, are annotated. (B) Active site of the Y34A crystal structure, the hydrogen bonding relay is maintained and depicted in pale cyan. (C) Active site of Y34H crystal structure, with hydrogen bonding relay and distances, in Å, annotated. (D) Superimposition of the active site chains of the human wild-type MnSOD (yellow) and Y34V (blue). Replacement of Tyr34 with Val interrupts the hydrogen bond network. The Y34V waters are depicted in red (wild-type waters in yellow), and the hydrogen bond relay of Y34V MnSOD is depicted by blue spheres, and bond distances, in Å, are annotated.