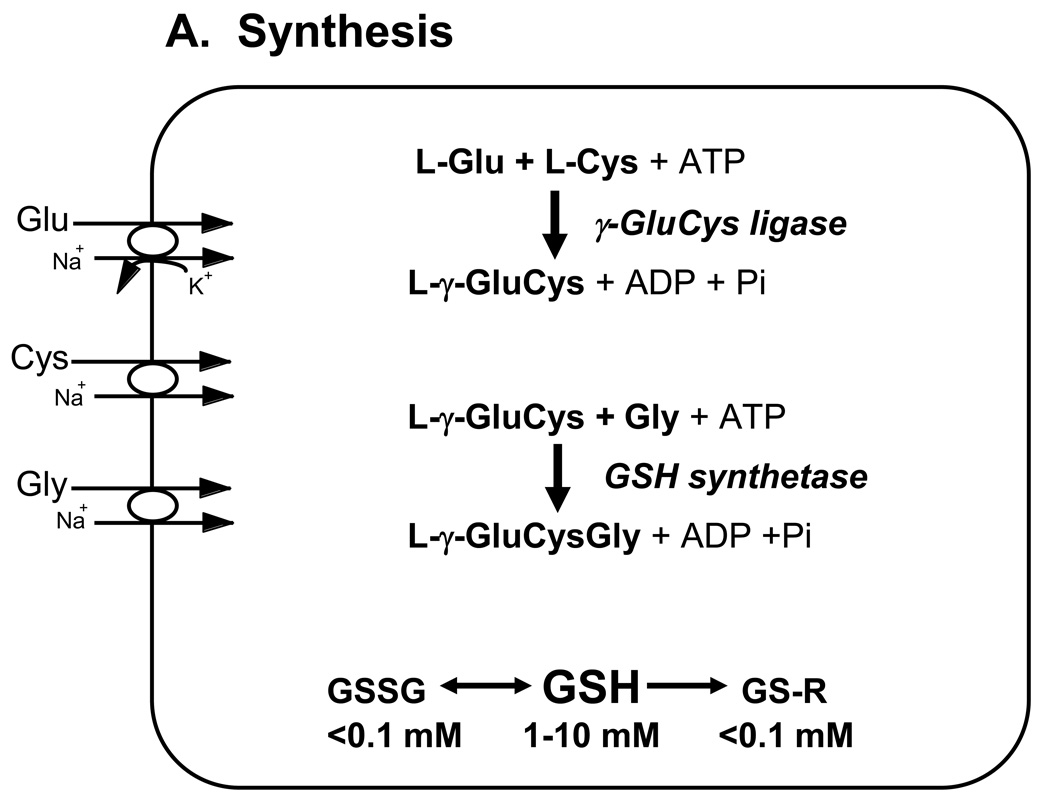
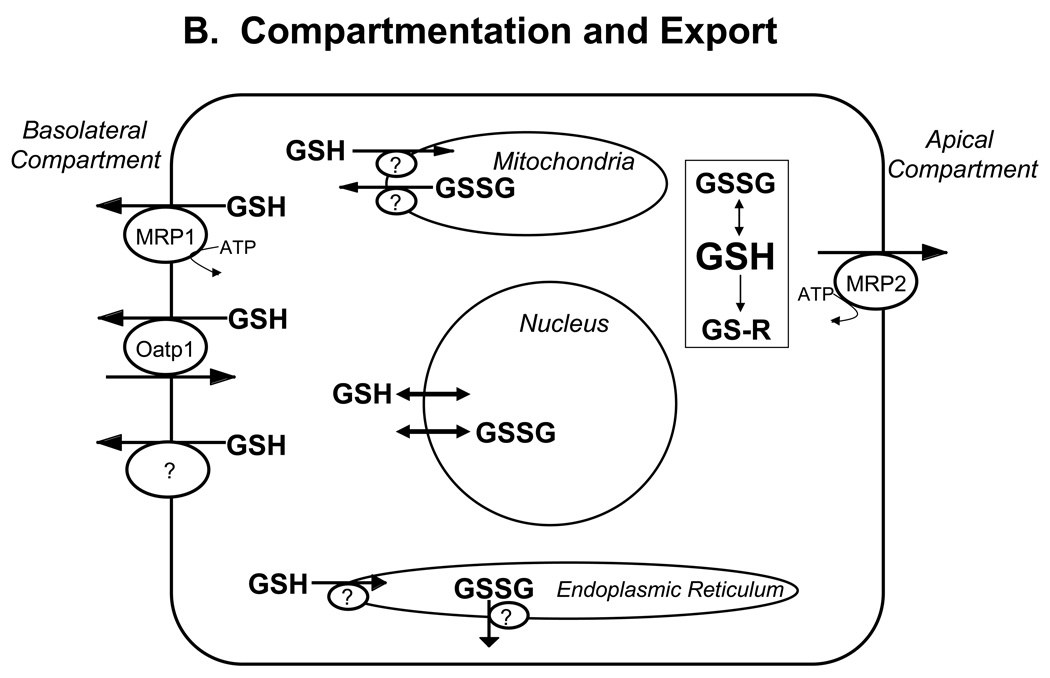
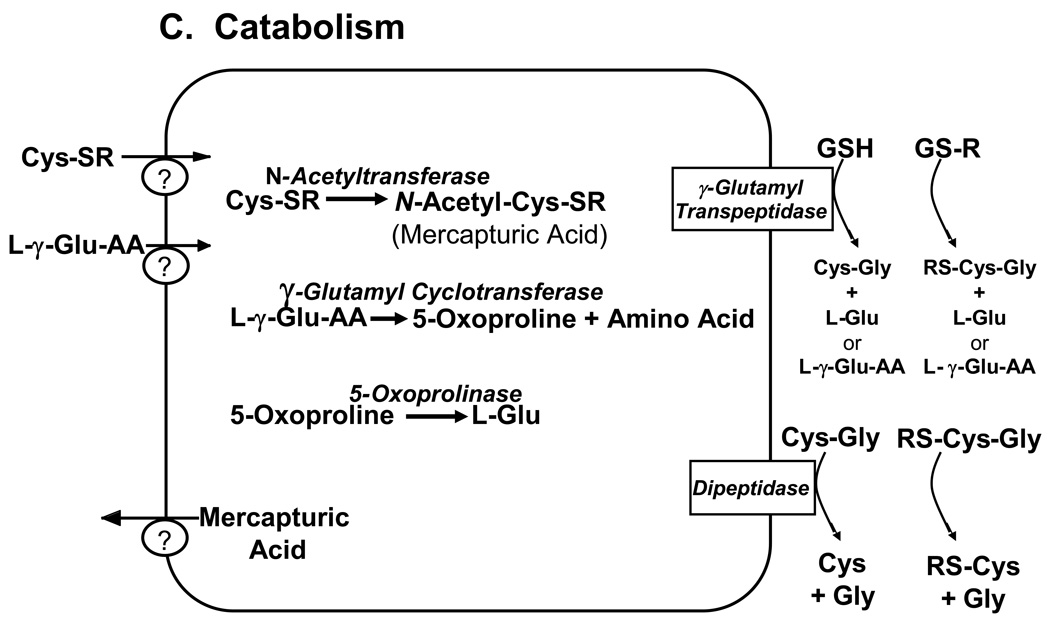
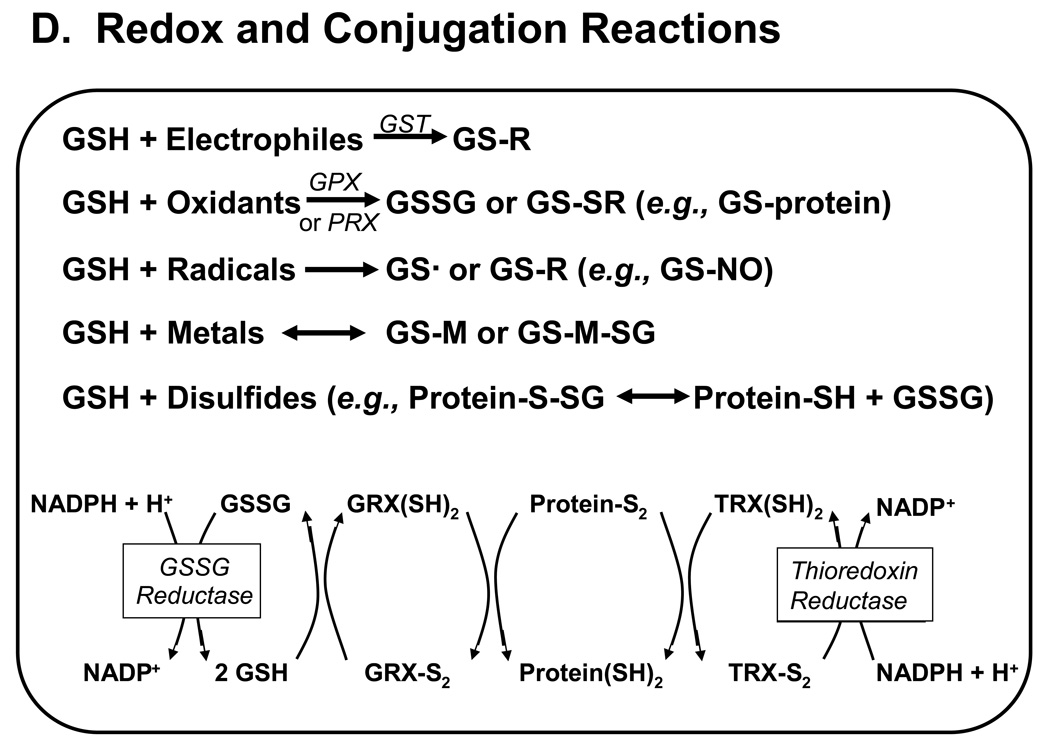
Figure 1. Major pathways of glutathione homeostasis in mammalian cells.
(A) GSH is synthesized in the cell cytosol from its precursor amino acids, glutamate, cysteine and glycine. Within the cell, it exists mainly (>98%) in the thiol-reduced form (GSH), but some is also present as glutathione disulfide (GSSG), and as glutathione conjugates (GS-R). (B) After its synthesis, some of the GSH is delivered into specific intracellular compartments, including mitochondria and endoplasmic reticulum, but much of the GSH is delivered to extracellular spaces, including blood plasma, exocrine secretions, lung lining fluid, and cerebrospinal fluid. In polarized cells, transport of GSH and its conjugates across the apical membrane is mediated largely by MRP2, whereas MRP1 and rat Oatp1 may contribute to GSH efflux across the basolateral plasma membrane into blood plasma, although the specific proteins that mediate GSH export and intracellular compartmentation remain poorly defined. (C) In contrast to GSH synthesis, which occurs intracellularly, GSH degradation occurs exclusively in the extracellular space, and only on the surface of cells that express the ectoenzyme γ-glutamyl transpeptidase. This enzyme is found on the apical membrane of many epithelial cells, but is also abundant at other key sites, including the kidney basolateral compartment. Once GSH and GSH-containing compounds are exported from cells, there are efficient intra- and inter-organ cycles of glutathione degradation and utilization consisting of: (a) extensive catabolism within apical spaces (e.g., bile and renal tubular fluid), as well as within sinusoidal compartments of some species; (b) cellular re-uptake of some of the breakdown products; and (c) intracellular utilization of these breakdown products, or conversion of cysteine S-conjugates (Cys-SR) to mercapturic acids, i.e., N-acetylcysteine S-conjugates (N-acetyl-Cys-SR). As illustrated in this panel, catabolism of glutathione S-conjugates (GS-R) leads to the formation of cysteine S-conjugates (Cys-SR). Cys-SRs are transported back into cells, where they may be substrates for the N-acetyltransferases, to generate N-acetyl-Cys-SRs, or mercapturic acids. Mercapturic acids are exported from cells for eventual elimination in urine or feces, but the transport mechanisms are not clearly defined. Note that γ-glutamyl transpeptidase can catalyze either hydrolyses reactions, to release free L-Glu, or transpeptidation reactions, to lead to the formation of γ-Glu bound to either amino acids or peptides (γ-Glu-AA). These γ-Glu-AAs can be transported back into cells, where they are substrates for the enzyme γ-glutamyl cyclotransferase: this enzyme generates 5-oxoproline and releases the amino acid or peptide that is bound to L-Glu. 5-Oxoproline is converted to L-Glu by the ATP-requiring enzyme 5-oxoprolinase. (D) GSH is a cofactor, coenzyme, and/or substrate for a number of enzymes, and can participate in a number of redox and conjugation reactions. Notably GSH can react with many electrophilic chemicals to generate glutathione S-conjugates (GS-R). Although conjugation reactions can occur spontaneously, these reactions are often catalyzed by the glutathione S-transferases (GST). GSH also facilitates the reduction of oxidants via glutathione peroxidases (GPX), and is involved in maintaining the redox state of protein thiols via the enzymes glutaredoxins (GRX), thioredoxins (TRX), and peroxiredoxins (PRX).