Abstract
Coxiella burnetii is an obligate intracellular bacterial pathogen that causes the zoonosis Q fever. While an effective whole-cell vaccine against Q fever exists, the vaccine has limitations in being highly reactogenic in sensitized individuals. Thus, a safe and effective vaccine based on recombinant protein antigen (Ag) is desirable. To achieve this goal, a better understanding of the host response to primary infection and the precise mechanisms involved in protective immunity to C. burnetii are needed. This review summarizes our current understanding of adaptive immunity to C. burnetii with a focus on recent developments in the field.
Keywords: Adaptive Immunity, Coxiella burnetii, Cell-mediated Immunity, Antibody-mediated Immunity, Dendritic Cell, Vaccine
Introduction
Coxiella burnetii, an obligate intracellular gram negative bacterium with near world-wide distribution, is the causative agent of the zoonosis Q fever. Roughly half of C. burnetii infections are asymptomatic, but result in seroconversion. Clinical Q fever can present as two basic forms: acute or chronic. Acute Q fever normally manifests as a self-limiting flu-like illness characterized by high-grade fever, peri-orbital headache and myalgia (1). However, in some cases pneumonia occurs requiring hospitalization. C. burnetii can establish a persistent, latent infection that may reactivate months or years after initial exposure to the organism to cause chronic disease. Chronic Q fever is typically associated with patients who are immunocompromised and/or who have pre-existing heart valve defects and most commonly presents as endocarditis (1). Because of a very low infectious dose, stability in the environment, and an aerosol route of transmission, C. burnetii is considered a potential biological weapon. Consequently, C. burnetii is categorized by the Centers for Disease Control and Prevention as a category B Select Agent.
Cattle, sheep and goats are major reservoirs of C. burnetii. The organism can be found in the milk, urine and feces of these animals as well as the placenta and birth fluids. Humans are most commonly infected through inhalation of contaminated dust or aerosols generated by livestock operations involving these animals. Consequently, Q fever is an occupational hazard for veterinarians, abattoir workers, dairy farmers and anyone with regular contact with livestock or their products (1). Urban outbreaks of Q fever are also associated with contact with infected domestic cats (2).
Currently, there is no FDA-approved Q fever vaccine available in the United States. An effective vaccine (Q-vax®, Commonwealth Serum Laboratories) has been developed by an Australian company and is commonly used in Australia. Q-vax consists of formalin-inactivated C. burnetii and provides long-lived protective immunity with a single dose (3). However, because of negative side effects in previously sensitized individuals, potential vaccinees require pre-vaccination skin testing. A better understanding of adaptive immunity to C. burnetii would help progress towards a safe and effective vaccine that does not require pre-screening. Herein, we have summarized the current state of C. burnetii immunology with a focus on specific areas in need of further study to advance our knowledge of the adaptive immune response to C. burnetii.
C. burnetii-Host Cell Interactions
C. burnetii typically infects humans via the aerosol route and alveolar macrophages (aMΦ) and other mononuclear phagocytes are believed to be the primary target cells of the pathogen. The bacteria are engulfed by MΦ and are retained in a phagosomal compartment that matures to acquire many characteristics of a secondary lysosome (4). Unlike other intracellular pathogens, such as Mycobacterium spp. and Legionella pneumophila, C. burnetii does not subvert phagosome-lysosome fusion to create a replicative niche (5). In fact, C. burnetii requires the moderately acidic pH (<5) of this compartment for its metabolism and subsequent replication (6). As mononuclear phagocytes are typically responsible for phagocytosis and killing of invading pathogens, the fact that C. burnetii prefers to reside in phagolysomes within these cells presents some interesting problems for the host immune system.
Differential trafficking of virulent phase I and avirulent phase II C. burnetii has been proposed (7, 8). Virulent phase I strains are always isolated from an infected animal or patient and produce a full-length lipopolysaccharide (LPS) (9, 10). Repeated passage of phase I organisms in vitro results in conversion to a phase II phenotype where bacteria produce a truncated LPS molecule lacking the terminal O-antigen sugars (9, 10). Phase II C. burnetii are severely attenuated and cannot establish an infection in an immunocompetent host (11). There are several potential explanations for the attenuation of phase II C. burnetii. Phase II organisms are more sensitive to complement-mediated lysis than fully virulent phase I strains (12). Phase II C. burnetii are also believed to engage different receptors on monocytes and macrophages that may result in differential uptake, trafficking and intracellular replication between phase II and phase I bacteria (7, 8).
Virulent C. burnetii productively infect mononuclear phagocytes in vivo and these cells appear unable to control bacterial growth in naive animals. Interestingly, full-length phase I LPS from C. burnetii does not stimulate macrophages and may actually be a TLR4 antagonist (13). While C. burnetii does not appear to signal through TLR4, Honstettre et al. (14) have reported that TLR4 does participate in bacterial uptake. However, TLR4 knockout mice do not appear to be deficient in their ability to control C. burnetii infection (14). Zamboni et al. demonstrated that avirulent C. burnetii stimulate macrophages through TLR2 (13). However, given the low infectious dose of C. burnetii (less than 10 viable organisms (11)), the innate immune system appears unable to contain primary infection by this organism in a large number of exposed individuals.
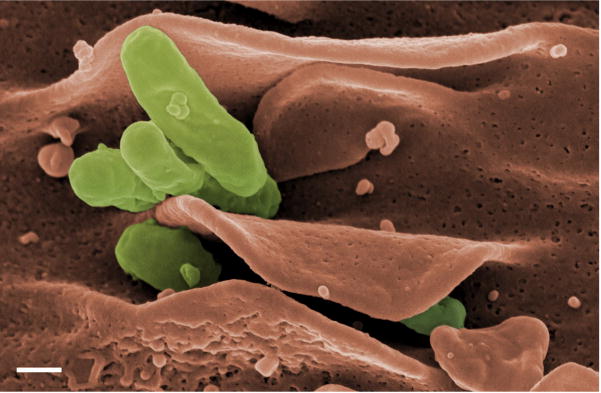
Dendritic cells (DC) serve as immune sentinels that detect the presence of pathogens and orchestrate the host’s immune response to infection (15). Because of their phagocytic nature and prevalence in mucosal tissues, immature DC are likely one of the first cell types encountered by C. burnetii during natural infection. Phase I C. burnetii can infect and grow within human DC without inducing maturation or inflammatory cytokine production by these cells (Fig. 1) (16). In contrast, phase II bacteria, with their truncated LPS, induce dramatic maturation and inflammatory cytokine production. Interestingly, we found that full-length C. burnetii LPS is required for the organism to avoid DC stimulation (16). These results suggest a novel role for LPS as a shielding molecule that prevents access of C. burnetii surface molecules to pattern recognition receptors on DC. Additionally, a lack of DC maturation in response to virulent C. burnetii would likely result in presentation of bacterial Ag by unstimulated, steady-state DC in vivo. The net effect of this might be the induction of tolerance to C. burnetii Ag which, if true, would be important in the establishment of persistent infection (17, 18).
Fig. 1.
Scanning electron micrograph showing internalization of the Nine Mile phase I strain of C. burnetii by human monocyte-derived DC. Bacteria are pseudocolored green while the DC is pseudocolored orange. Bar, 0.2 μm.
Cell-mediated Immunity to C. burnetii
Most research on the immune response to C. burnetii infection can be divided into two main areas of study: 1) immune response to primary infection and, 2) protection against challenge after vaccination.
The importance of cell-mediated immunity (CMI) in defense against a number of intracellular pathogens, including Mycobacterium tuberculosis, Legionella pneumophila, and Listeria monocytogenes, is well established (reviewed in (19–21)). Uptake of intracellular pathogens by antigen presenting cells (APC) leads to presentation of pathogen Ag on the surface of the host cell. This Ag presentation is typically accompanied by expression of T cell costimulatory molecules on the APC surface. Cumulatively, these events lead to mobilization of Ag-specific T cells. Large numbers of activated T cells are recruited to sites of infection where they produce inflammatory cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor (TNF). These cytokines stimulate an antimicrobial response in a variety of cell types thereby controlling infection. T cells can also recruit mononuclear cells, lymphocytes and fibroblasts to sites of infection and establish granulomas, effectively walling-off invading pathogens. C. burnetii infection can stimulate a strong cellular host response. Lymphocytes from vaccinated and convalescent humans proliferate and produce IFN-γ in response to C. burnetii Ag (22–24). IFN-γ stimulation allows monocytes/macrophages and fibroblasts to control C. burnetii growth (8, 25–27). The production of reactive oxygen and nitrogen species by cells in response to IFN-γ appears to play an import role in controlling intracellular bacterial replication (25, 27). C. burnetii infection often results in granulomatous lesions in a variety of different tissues including, but not limited to, the spleen, liver and lungs. Chronic Q fever is associated with reduced granuloma formation (28). Thus, cell-mediated immunity is important for control of acute infection and prevention of disease reactivation.
Andoh et al. (29) recently described a detailed study of the primary immune response to C. burnetii infection using a mouse model of acute Q fever. They found that T cell-deficient and IFN-γ k/o mice show greatly increased susceptibility to C. burnetii infection. Thus, it would appear that T cells are required for resolution of disease, most likely because they serve as the main source of IFN-γ. So far, the roles of specific populations of T cells, such as CD4+ or CD8+, have not been determined. Sidwell et al. demonstrated in a series of papers that suppression of CMI in mice caused by pregnancy, corticosteroid treatment or gamma-irradiation causes reactivation of persistent C. burnetii infection (30–32). Thus, cellular immunity is clearly important in the control of C. burnetii infection.
To evaluate potential mechanisms of protective immunity, Zhang et al. studied protection of vaccinated mice against challenge with virulent C. burnetii (33). They found that vaccination with formalin-fixed phase I or phase II bacteria induced a predominantly Th1-type immune response. However, only vaccination with phase I organism provided protection. Splenocytes from phase I vaccinated mice are protective when adoptively transferred into naive mice indicating a role for the cellular response in the protection elicited by vaccination (33). Thus, CMI plays an important role in protective immunity elicited by vaccination.
Humoral Immunity to C. burnetii
Historically, antibody-mediated immunity was considered important for protection against extracellular pathogens whereas immunity to intracellular pathogens was thought to be exclusively cell-mediated. Recently it has become clear that this paradigm is not accurate and that antibody plays an important role in protective immunity to a number of intracellular pathogens (34). Antibody (Ab) can mediate protection via a variety of different mechanisms. These include direct bactericidal activity, complement activation, toxin neutralization, opsonizaton/phagocytosis, antibody-dependent cellular cytotoxicity (ADCC), altered intracellular trafficking of pathogens, and modulation of the immune response through interactions with Fc and complement receptors. Most of the effective vaccines that have been developed to date exert their protective effects through Ab-dependent mechanisms. Thus, a better understanding of Ab-mediated immunity (AMI) to C. burnetii will be important for vaccine development.
Ab develops within 3–4 weeks of onset of symptoms of acute Q fever, mostly against phase II Ag which are considered proteinaceous (35). In fact, development of anti-phase II Ab accompanied by low levels of anti-phase I Ab, which are primarily directed against LPS, is considered diagnostic of acute Q fever. In chronic Q fever patients the trend is reversed where an anti-phase I titer of >800 is considered diagnostic (35). The importance of Ab in immunity to C. burnetii has been overlooked in the past as not all vaccinated or convalescent patients have detectable levels of serum Ab (3). However, as the sensitivity of methods used to detect Ab has increased, so has the percentage of individuals testing positive in human studies (3). Because the levels of Ab necessary for protective immunity in humans are unknown, we must consider the role of Ab when designing subunit vaccines.
Shortly after the discovery of C. burnetii, Burnet and Freeman described the passive protection of mice and guinea pigs with C. burnetii antiserum (36, 37). The fact that antibody can protect a naive animal against this obligate intracellular pathogen raises some interesting questions. For example, at what point during infection in vivo do antibodies gain access to C. burnetii? Furthermore, how does antibody opsonization affect the replication of a pathogen that normally resides in the phagolysosome of a macrophage?
Ab-opsonization of phase I C. burnetii has been reported to increase phagocytosis of the organism by MΦ (38). Indeed, we have observed dramatically increased uptake of Ab-opsonized C. burnetii by human monocytes, MΦ and DC in vitro. Interestingly, Ab-opsonization had no effect on the growth rate of bacteria in these cells (Shannon and Heinzen, unpublished data). Thus, increased phagocytosis and altered trafficking are probably not mechanisms of AMI to C. burnetii.
We have found that infection of DC with Ab opsonized C. burnetii results in increased expression of maturation markers and inflammatory cytokine production (Shannon and Heinzen, unpublished data). This effect was Fc receptor (FcR)-dependent as evidenced by a reduced response of DC from FcR knockout compared to C57Bl/6 mice. However, wild-type and FcR knockout mice are equally protected by passive immunization indicating that FcR are not essential for AMI in vivo (Shannon and Heinzen, unpublished data). We have also investigated the role of complement in AMI to C. burnetii by passively immunizing complement-deficient mice. We found that, like FcR, complement is not essential for passive protection in vivo (Shannon and Heinzen, unpublished data). Work is in progress to determine the precise immune mechanisms responsible for passive immunity to C. burnetii.
Andoh et al. report that B cell-deficient mice display more pathology than wild-type mice after C. burnetii infection indicating Ab may play a role in reducing inflammatory tissue damage after containment of infection (29). However, antibody does not appear essential for resolution of primary infection. Passive immunization of naive mice with serum from vaccinated mice protects against challenge (33, 39). Thus, antibody can provide complete protection in the mouse model. Interestingly, athymic mice are not protected by passive immunization indicating that T cells are required for antibody-mediated immunity to C. burnetii (39). In support of this, Zhang et al. recently demonstrated that passive immunization with immune serum does not protect SCID mice, while adoptive transfer of T cells from immune animals does, indicating that T cell-mediated immunity is essential for protective immunity to C. burnetii (33).
It is clear that AMI plays an important role in C. burnetii infection but the precise mechanisms by which it contributes to immunity have not been determined. A better understanding of the role of AMI in protection against infection will be necessary if an effective subunit Q fever vaccine is to be developed.
Q Fever Vaccine Development
Currently, there is no FDA-approved Q fever vaccine available in the United States. Several different vaccine formulations have been developed in the past each with varying degrees of effectiveness. Chloroform:methanol residue (CMR) extracts of phase I C. burnetii have been explored for use as Q fever vaccines. These extracts are less reactogenic in animal models and thus, may be safer for sensitized individuals than the whole cell vaccines (WCV) (40, 41). Unlike with WCV, multiple doses of CMR can be administered without adverse effects (40, 42). Phase I clinical trials of the CMR formulation have been conducted and the vaccine appears to be safe and immunogenic in human volunteers with no history of C. burnetii exposure (42). The safety of this vaccine in skin test positive individuals still needs to be determined. Attempts have been made to develop phase II C. burnetii into a fixed WCV or live attenuated vaccine with minimal success. A live attenuated Q fever vaccine was developed in the Soviet Union from the phase II form of the M-44 strain of C. burnetii (43). However, this strain was shown to persist and cause pathologic changes in laboratory animals, which has raised concerns about its safety in humans (44). Ormsbee et al. showed that vaccination with formalin-inactivated phase I bacteria was 100 to 300 times more protective than vaccination with phase II organisms (45). The only known difference between phase I and phase II C. burnetii is the presence or absence of O-antigen, respectively. Therefore, full-length phase I LPS appears to be critical for the generation of robust anti-C. burnetii immunity. In support of this, vaccination of mice with purified phase I LPS is protective (33). However, due to the difficulty in obtaining 100% pure LPS one can always question whether or not small amounts of other Ag are contributing to immunity. Interestingly, Abinanti and Marmion (46) showed a correlation between the ability of immune serum to protect mice and the amount of antibody against phase I Ag present in the serum. Whole-cell phase I vaccines have been proven protective in livestock, laboratory animals and humans (3, 41, 47–50). One of the most widely used and effective WCV is a preparation of the Henzerling strain of C. burnetii in phase I that has been formalin-inactivated. This vaccine, called Q-vax®, has been in widespread use in high-risk individuals in Australia for over 25 years. One 30 μg dose of Q-vax delivered subcutaneously provides long-lived immunity to Q fever (50, 51). However, individuals with previous exposure to C. burnetii can have a severe delayed-type hypersensitivity reaction to the vaccine. Thus, potential vaccinees need to be pre-screened by skin test to determine if they have had prior exposure. However, even with this pre-screening side effects such as headache and flu-like symptoms have been reported in 10–18% of vaccine recipients (50). Clearly a safe, effective subunit vaccine that does not require pre-screening would be desirable.
A number of potential protective protein Ag have been identified. A 67 kDa protein affinity purified from the QiYi strain of C. burnetii can provide 100% protection in guinea pigs and mice (52). Purified native P1, a 29 kDa major outer membrane protein, provides protection from challenge in mice (53). Zhang and Samuel (54) cloned four proteins (Com1, P1, Cb-Mip and P28) that were recognized by C. burnetii immune sera. However, vaccination of mice with these individual recombinant proteins failed to provide protection against challenge (54). The fact that purified native proteins protect, but recombinant proteins do not, suggests Ag epitopes on natively conformed proteins are important for protection or that the native protein preparations used in these studies were contaminated with small amounts of other protective Ag, most likely LPS. Interestingly, Li et al. showed that recombinant P1 or HspB were unable to induce protective immunity, but a recombinant P1-HspB fusion protein provided complete protection (55). Thus, candidate Ag for subunit vaccines may need to be combined to induce protective immunity to C. burnetii. A genetic library consisting of 97% of C. burnetii’s open reading frames (ORF) has been constructed. To date, ~75% of these clones have been individually expressed by in vitro transcription and translation and spotted on slides to create C. burnetii ORFeome microarrays (Beare et al., submitted). These protein arrays were probed with naive, immune and convalescent human sera and >50 immunoreactive proteins were identified, including a number with predicted outer membrane localization. We are currently pursuing the proteins on this list as potential subunit vaccine candidates.
Chronic C. burnetii Infection
Human exposure to C. burnetii can result in a variety of outcomes including severe flu-like symptoms to asymptomatic infection (1). Regardless of presentation, C. burnetii can establish a persistent, latent infection that may reactivate months or years after initial exposure and cause chronic disease. This reactivation is largely dependent on the host’s immune status as chronic Q fever is typically associated with some type of immune suppression (56, 57). One of the most frequent manifestations of chronic Q fever is endocarditis. In fact, C. burnetii is a leading cause of blood culture-negative endocarditis (58). Chronic Q fever can be treated with antibiotics; however, treatment does not result in clearance of the organism and patients often require life-long prophylactic antibiotic therapy to prevent reactivation (1). A vaccine that could be administered to persistently infected individuals to boost their immune system and drive the immune response towards elimination of the persistent bacteria would be advantageous for the treatment of chronic Q fever. While our knowledge of the immunology of chronic Q fever has steadily increased, a better understanding is needed before such a vaccine can be developed.
Elevated levels of interleukin-10 (IL-10) have been found in chronic Q fever patients and IL-10 has been implicated in the ability of C. burnetii to establish a persistent infection (59, 60). IL-10 is a pleiotropic cytokine exhibiting both pro- and anti-inflammatory properties. However, it is generally thought to play more of an anti-inflammatory role in most in vivo situations. IL-10 is essential for maintenance of the delicate balance between immunity to pathogens and pathology that can result when an immune response is left unchecked. A variety of pathogens (e.g. Mycobacterium tuberculosis, Leishmania sp. and Helicobacter pylori) can take advantage of the anti-inflammatory properties of IL-10 as a means of avoiding sterilizing immunity and establishing a persistent infection (reviewed in (61–63)). Several lines of evidence point to an important role for IL-10 in chronic Q fever. Capo et al. found that peripheral blood mononuclear cells (PBMC) from chronic disease patients spontaneously produce elevated levels of IL-10 and that the amount of IL-10 produced is related to disease severity and likelihood of relapse (59). Furthermore, Honstettre et al. monitored cytokine production by PBMC from acute disease patients and found a correlation between IL-10 levels and progression of the patient to chronic disease (60). IL-10 induces growth of C. burnetii in human monocytes and monocytes from chronic Q fever patients are more permissive for growth than controls (64). IL-10 may increase C. burnetii replication by inhibiting TNF production by these cells. C. burnetii establishes a more robust infection in transgenic mice overexpressing IL-10 in macrophages (macIL-10tg) and macrophages from these mice fail to kill the bacteria (65). These mice also develop very similar pathology to human chronic Q fever patients. Therefore, the macIL-10tg mouse may be an informative animal model of chronic Q fever.
Clearly IL-10 plays an important role in C. burnetii infection. Identifying the main source of this cytokine in vivo will be necessary for a more complete understanding of Q fever pathogenesis. Regulatory T cells can be a major source of IL-10 during infection (66). T cells from chronic Q fever patients can be unresponsive to C. burnetii Ag and this T cell suppression is associated with an Ag-specific population of “suppressor” T cells (67, 68). These results were published in the mid-1980s. In the intervening time the field of suppressor or regulatory T cells has exploded. The tools and techniques available for study of these cells in vivo and in vitro are now widely available. Thus, the role of regulatory T cells in Q fever needs to be investigated using modern approaches.
Conclusions
As an obligate intracellular pathogen, C. burnetii presents some interesting challenges to immunologists. The organism takes up residence in a lysosome-like compartment in a phagocyte. Despite this intracellular niche, passive immunization with antiserum provides complete protection against C. burnetii infection. Interestingly, T cells are absolutely required for control of primary infection and passive protection provided by Ab. However, to date, we have been unable to determine the precise mechanism(s) of AMI to C. burnetii. An efficacious whole-cell vaccine against Q fever has been developed, although the exact mechanisms by which this vaccine provides protection are only partially understood. The recent developments in the field of C. burnetii immunology have undoubtedly moved us closer to the goal of a safe, effective subunit vaccine for Q fever. However, further studies on the basic mechanisms of immunity to this pathogen are needed.
Acknowledgments
We thank Shelly Robertson for critical review of the manuscript, Dave Dorward for scanning electron microscopy, and Anita Mora for graphic illustrations.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergyand Infectious Diseases.
References
- 1.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12(4):518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comer JA, Paddock CD, Childs JE. Urban zoonoses caused by Bartonella, Coxiella, Ehrlichia, and Rickettsia species. Vector Borne Zoonotic Dis. 2001;1(2):91–118. doi: 10.1089/153036601316977714. [DOI] [PubMed] [Google Scholar]
- 3.Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick DA, Izzo AA, Esterman A, Feery B, Shapiro RA. Vaccine prophylaxis of abattoir-associated Q fever: Eight years’ experience in australian abattoirs. Epidemiol Infect. 1990;104(2):275–287. doi: 10.1017/s0950268800059458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voth DE, Heinzen RA. Lounging in a lysosome: The intracellular lifestyle of Coxiella burnetii. Cell Microbiol. 2007;9(4):829–840. doi: 10.1111/j.1462-5822.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 5.Sauer JD, Shannon JG, Howe D, Hayes SF, Swanson MS, Heinzen RA. Specificity of Legionella pneumophila and Coxiella burnetii vacuoles and versatility of Legionella pneumophila revealed by coinfection. Infect Immun. 2005;73(8):4494–4504. doi: 10.1128/IAI.73.8.4494-4504.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackstadt T, Williams JC. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capo C, Lindberg FP, Meconi S, Zaffran Y, Tardei G, Brown EJ, Raoult D, Mege JL. Subversion of monocyte functions by Coxiella burnetii: Impairment of the cross-talk between alphaVbeta3 integrin and CR3. J Immunol. 1999;163(11):6078–6085. [PubMed] [Google Scholar]
- 8.Ghigo E, Capo C, Tung CH, Raoult D, Gorvel JP, Mege JL. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J Immunol. 2002;169(8):4488–4495. doi: 10.4049/jimmunol.169.8.4488. [DOI] [PubMed] [Google Scholar]
- 9.Hackstadt T. Antigenic variation in the phase I lipopolysaccharide of Coxiella burnetii isolates. Infect Immun. 1986;52(1):337–340. doi: 10.1128/iai.52.1.337-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackstadt T, Peacock MG, Hitchcock PJ, Cole RL. Lipopolysaccharide variation in Coxiella burnettii: Intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985;48(2):359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moos A, Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun. 1987;55(5):1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vishwanath S, Hackstadt T. Lipopolysaccharide phase variation determines the complement-mediated serum susceptibility of Coxiella burnetii. Infect Immun. 1988;56(1):40–44. doi: 10.1128/iai.56.1.40-44.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamboni DS, Campos MA, Torrecilhas AC, Kiss K, Samuel JE, Golenbock DT, Lauw FN, Roy CR, Almeida IC, Gazzinelli RT. Stimulation of toll-like receptor 2 by Coxiella burnetii is required for macrophage production of pro-inflammatory cytokines and resistance to infection. J Biol Chem. 2004 doi: 10.1074/jbc.M410340200. [DOI] [PubMed] [Google Scholar]
- 14.Honstettre A, Ghigo E, Moynault A, Capo C, Toman R, Akira S, Takeuchi O, Lepidi H, Raoult D, Mege JL. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through toll-like receptor 4. J Immunol. 2004;172(6):3695–3703. doi: 10.4049/jimmunol.172.6.3695. [DOI] [PubMed] [Google Scholar]
- 15.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 16.Shannon JG, Howe D, Heinzen RA. Virulent Coxiella burnetii does not activate human dendritic cells: Role of lipopolysaccharide as a shielding molecule. Proc Natl Acad Sci U S A. 2005;102(24):8722–8727. doi: 10.1073/pnas.0501863102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99(1):351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FoxP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur J Immunol. 2004;34(3):762–772. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 19.Neild AL, Roy CR. Immunity to vacuolar pathogens: What can we learn from Legionella? Cell Microbiol. 2004;6(11):1011–1018. doi: 10.1111/j.1462-5822.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 20.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4(10):812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 21.Reece ST, Kaufmann SH. Rational design of vaccines against tuberculosis directed by basic immunology. Int J Med Microbiol. 2008;298(1–2):143–150. doi: 10.1016/j.ijmm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Izzo AA, Marmion BP, Hackstadt T. Analysis of the cells involved in the lymphoproliferative response to Coxiella burnetii antigens. Clin Exp Immunol. 1991;85(1):98–108. doi: 10.1111/j.1365-2249.1991.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzo AA, Marmion BP, Worswick DA. Markers of cell-mediated immunity after vaccination with an inactivated, whole-cell Q fever vaccine. J Infect Dis. 1988;157(4):781–789. doi: 10.1093/infdis/157.4.781. [DOI] [PubMed] [Google Scholar]
- 24.Hinrichs DJ, Jerrells TR. In vitro evaluation of immunity to Coxiella burnetii. J Immunol. 1976;117(3):996–1003. [PubMed] [Google Scholar]
- 25.Brennan RE, Russell K, Zhang G, Samuel JE. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect Immun. 2004;72(11):6666–6675. doi: 10.1128/IAI.72.11.6666-6675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turco J, Thompson HA, Winkler HH. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun. 1984;45(3):781–783. doi: 10.1128/iai.45.3.781-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe D, Barrows LF, Lindstrom NM, Heinzen RA. Nitric oxide inhibits Coxiella burnetii replication and parasitophorous vacuole maturation. Infect Immun. 2002;70(9):5140–5147. doi: 10.1128/IAI.70.9.5140-5147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5(4):219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 29.Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect Immun. 2007;75(7):3245–3255. doi: 10.1128/IAI.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidwell RW, Gebhardt LP. Studies of latent Q fever infections. 3. Effects of parturition upon latently infected guinea pigs and white mice. Am J Epidemiol. 1966;84(1):132–137. doi: 10.1093/oxfordjournals.aje.a120618. [DOI] [PubMed] [Google Scholar]
- 31.Sidwell RW, Thorpe BD, Gebhardt LP. Studies on latent Q fever infections. I. Effects of whole body x-irradiation upon latently infected guinea pigs, white mice and deer mice. Am J Hyg. 1964;79:113–124. [PubMed] [Google Scholar]
- 32.Sidwell RW, Thorpe BD, Gebhardt LP. Studies of latent Q fever infections. II. Effects of multiple cortisone injections. Am J Hyg. 1964;79:320–327. doi: 10.1093/oxfordjournals.aje.a120386. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, Samuel JE. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in balb/c mice. J Immunol. 2007;179(12):8372–8380. doi: 10.4049/jimmunol.179.12.8372. [DOI] [PubMed] [Google Scholar]
- 34.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 35.Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36(7):1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnet FM, Freeman M. “Q” Fever: Factors affecting the appearance of Rickettsiae in mice. Med J Aust. 1938;2:1114–1116. [Google Scholar]
- 37.Burnet FM, Freeman M. Studies on the x strain (dyer) of Rickettsia burneti. II. Guinea pig infections with special reference to immunological phenomena. J Immunol. 1941;40:421–436. [Google Scholar]
- 38.Kazar J, Skultetyova E, Brezina R. Phagocytosis of Coxiella burneti by macrophages. Acta Virol. 1975;19(5):426–431. [PubMed] [Google Scholar]
- 39.Humphres RC, Hinrichs DJ. Role of antibody in Coxiella burnetii infection. Infect Immun. 1981;31(2):641–645. doi: 10.1128/iai.31.2.641-645.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams JC, Peacock MG, Waag DM, Kent G, England MJ, Nelson G, Stephenson EH. Vaccines against coxiellosis and Q fever. Development of a chloroform:methanol residue subunit of phase I Coxiella burnetii for the immunization of animals. Ann N Y Acad Sci. 1992;653:88–111. doi: 10.1111/j.1749-6632.1992.tb19633.x. [DOI] [PubMed] [Google Scholar]
- 41.Brooks DL, Ermel RW, Franti CE, Ruppanner R, Behymer DE, Williams JC, Stephenson EH. Q fever vaccination of sheep: Challenge of immunity in ewes. Am J Vet Res. 1986;47(6):1235–1238. [PubMed] [Google Scholar]
- 42.Waag DM, England MJ, Bolt CR, Williams JC. Phase 1 clinical trial of CMR Q fever vaccine: Low-dose priming enhances humoral and cellular immune responses to Coxiella burnetii. Clin Vaccine Immunol. 2008 doi: 10.1128/CVI.00119-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genig VA. A live vaccine 1-M-44 against Q-fever for oral use. J Hyg Epidemiol Microbiol Immunol. 1968;12(3):265–273. [PubMed] [Google Scholar]
- 44.Johnson JW, McLeod CG, Stookey JL, Higbee GA, Pedersen CE., Jr Lesions in guinea pigs infected with Coxiella burnetii strain M-44. J Infect Dis. 1977;135(6):995–998. doi: 10.1093/infdis/135.6.995. [DOI] [PubMed] [Google Scholar]
- 45.Ormsbee RA, Bell EJ, Lackman DB, Tallent G. The influence of phase on the protective potency of Q fever vaccine. J Immunol. 1964;92:404–412. [PubMed] [Google Scholar]
- 46.Abinanti FR, Marmion BP. Protective or neutralizing antibody in Q fever. Am J Hyg. 1957;66(2):173–195. doi: 10.1093/oxfordjournals.aje.a119894. [DOI] [PubMed] [Google Scholar]
- 47.Behymer DE, Biberstein EL, Riemann HP, Franti CE, Sawyer M, Ruppanner R, Crenshaw GL. Q fever (Coxiella burnetii) investigations in dairy cattle: Challenge of immunity after vaccination. Am J Vet Res. 1976;37(6):631–634. [PubMed] [Google Scholar]
- 48.Biberstein EL, Riemann HP, Franti CE, Behymer DE, Ruppanner R, Bushnell R, Crenshaw G. Vaccination of dairy cattle against Q fever (Coxiella burneti): Results of field trials. Am J Vet Res. 1977;38(2):189–193. [PubMed] [Google Scholar]
- 49.Waag DM, Kende M, Damrow TA, Wood OL, Williams JC. Injection of inactivated phase I Coxiella burnetii increases non-specific resistance to infection and stimulates lymphokine production in mice. Ann N Y Acad Sci. 1990;590:203–214. doi: 10.1111/j.1749-6632.1990.tb42221.x. [DOI] [PubMed] [Google Scholar]
- 50.Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick D, Cameron S, Esterman A, Feery B, Collins W. Vaccine prophylaxis of abattoir-associated Q fever. Lancet. 1984;2(8417–18):1411–1414. doi: 10.1016/s0140-6736(84)91617-9. [DOI] [PubMed] [Google Scholar]
- 51.Ackland JR, Worswick DA, Marmion BP. Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-vax (CSL) 1985–1990. Med J Aust. 1994;160(11):704–708. [PubMed] [Google Scholar]
- 52.Zhang YX, Zhi N, Yu SR, Li QJ, Yu GQ, Zhang X. Protective immunity induced by 67 k outer membrane protein of phase I Coxiella burnetii in mice and guinea pigs. Acta Virol. 1994;38(6):327–332. [PubMed] [Google Scholar]
- 53.Williams JC, Hoover TA, Waag DM, Banerjee-Bhatnagar N, Bolt CR, Scott GH. Antigenic structure of Coxiella burnetii. A comparison of lipopolysaccharide and protein antigens as vaccines against Q fever. Ann N Y Acad Sci. 1990;590:370–380. doi: 10.1111/j.1749-6632.1990.tb42243.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang GQ, Samuel JE. Identification and cloning potentially protective antigens of Coxiella burnetii using sera from mice experimentally infected with Nine Mile phase I. Ann N Y Acad Sci. 2003;990:510–520. doi: 10.1111/j.1749-6632.2003.tb07420.x. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Niu D, Wen B, Chen M, Qiu L, Zhang J. Protective immunity against Q fever induced with a recombinant P1 antigen fused with hspB of Coxiella burnetii. Ann N Y Acad Sci. 2005;1063:130–142. doi: 10.1196/annals.1355.021. [DOI] [PubMed] [Google Scholar]
- 56.Boschini A, Di Perri G, Legnani D, Fabbri P, Ballarini P, Zucconi R, Boros S, Rezza G. Consecutive epidemics of Q fever in a residential facility for drug abusers: Impact on persons with human immunodeficiency virus infection. Clin Infect Dis. 1999;28(4):866–872. doi: 10.1086/515192. [DOI] [PubMed] [Google Scholar]
- 57.Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33(3):312–316. doi: 10.1086/321889. [DOI] [PubMed] [Google Scholar]
- 58.Brouqui P, Raoult D. New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol Med Microbiol. 2006;47(1):1–13. doi: 10.1111/j.1574-695X.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 59.Capo C, Zaffran Y, Zugun F, Houpikian P, Raoult D, Mege JL. Production of interleukin-10 and transforming growth factor beta by peripheral blood mononuclear cells in Q fever endocarditis. Infect Immun. 1996;64(10):4143–4147. doi: 10.1128/iai.64.10.4143-4147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Honstettre A, Imbert G, Ghigo E, Gouriet F, Capo C, Raoult D, Mege JL. Dysregulation of cytokines in acute Q fever: Role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. J Infect Dis. 2003;187(6):956–962. doi: 10.1086/368129. [DOI] [PubMed] [Google Scholar]
- 61.Aliberti J. Host persistence: Exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol. 2005;5(2):162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 62.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28(9):378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: Mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 2003;3(9):578–590. doi: 10.1016/s1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 64.Ghigo E, Capo C, Raoult D, Mege JL. Interleukin-10 stimulates Coxiella burnetii replication in human monocytes through tumor necrosis factor down-modulation: Role in microbicidal defect of Q fever. Infect Immun. 2001;69(4):2345–2352. doi: 10.1128/IAI.69.4.2345-2352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meghari S, Bechah Y, Capo C, Lepidi H, Raoult D, Murray PJ, Mege JL. Persistent Coxiella burnetii infection in mice overexpressing IL-10: An efficient model for chronic Q fever pathogenesis. PLoS Pathog. 2008;4(2):e23. doi: 10.1371/journal.ppat.0040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suvas S, Rouse BT. Treg control of antimicrobial T cell responses. Curr Opin Immunol. 2006;18(3):344–348. doi: 10.1016/j.coi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Koster FT, Williams JC, Goodwin JS. Cellular immunity in Q fever: Modulation of responsiveness by a suppressor T cell-monocyte circuit. J Immunol. 1985;135(2):1067–1072. [PubMed] [Google Scholar]
- 68.Koster FT, Williams JC, Goodwin JS. Cellular immunity in Q fever: Specific lymphocyte unresponsiveness in Q fever endocarditis. J Infect Dis. 1985;152(6):1283–1289. doi: 10.1093/infdis/152.6.1283. [DOI] [PubMed] [Google Scholar]