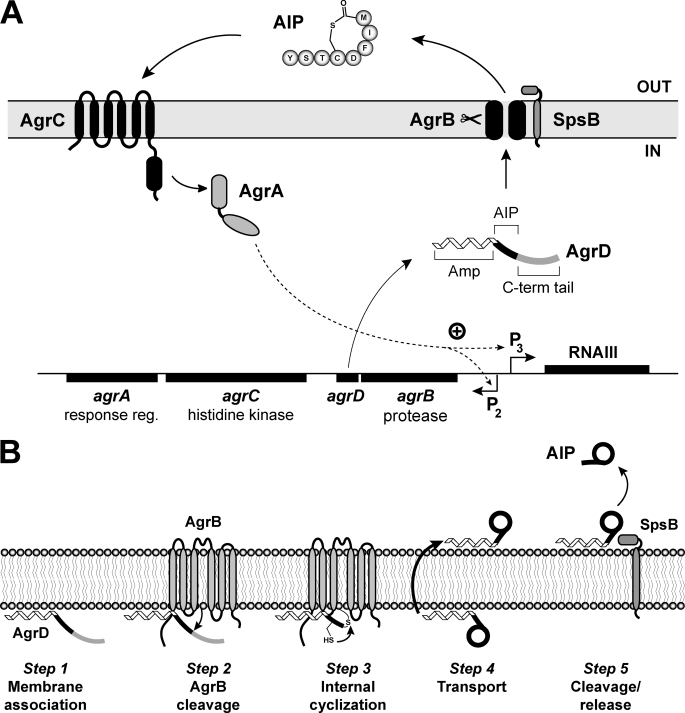
FIGURE 1.
Schematic of the S. aureus agr system and a model for AIP biosynthesis. A, chromosomal agr locus is composed of two divergently expressed transcripts. Promoter P2 drives expression of the agrBDCA operon. AgrD is the peptide precursor of AIP (type I is shown) that is composed of three parts, including an N-terminal amphipathic helix (Amp), a middle region that becomes AIP, and a C-terminal (C-term) charged tail. AgrB is an integral membrane endopeptidase essential for AIP production. AgrC is a membrane histidine kinase that has an extracellular receptor for AIP binding. AgrA is a response regulator and part of a two-component pair with AgrC. Activated AgrA induces transcription from promoters P2 and P3, and the P3 promoter drives expression of RNAIII, the primary effector of the agr system. SpsB is the housekeeping type I signal peptidase that completes the AIP biosynthetic pathway. B, AIP biosynthetic pathway model. Step 1, the N-terminal amphipathic helix associates with the cytoplasmic membrane. Step 2, AgrB removes the C-terminal tail of AgrD. Step 3, the remaining AgrD peptide fragment (N-terminal and AIP portion) is bound to AgrB as a thioester at AgrB residue Cys-84. The AgrD cysteine residue can attack the thioester bond displacing the peptide and forming the thiolactone ring. Step 4, the AIP precursor (N-terminal helix and AIP thiolactone) is transported to the outer face of the membrane. Step 5, SpsB removes the amphipathic helix, releasing AIP from the membrane.