Figure 3.
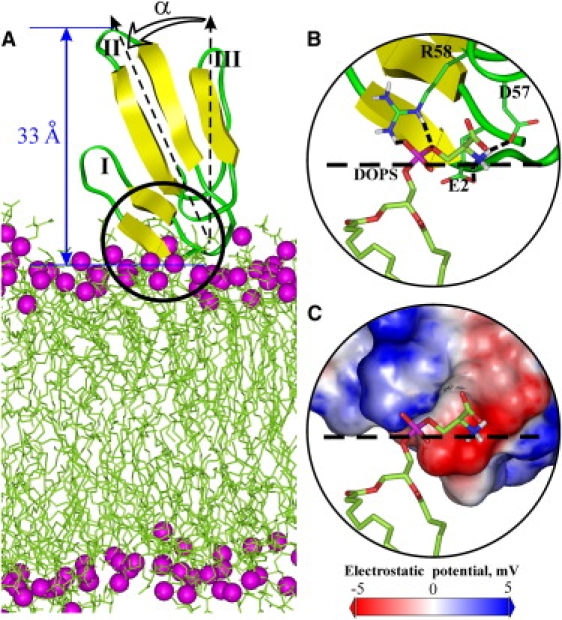
Topology of NTII on the membrane surface. (A) MD model of NTII association with an explicit DOPS bilayer. The angle α between the central loop of NTII (vector Thr-21 Cα −His-31 Cα) and the membrane normal is shown. The distance between phosphorus atoms (magenta balls) of lipids and the top tip of the toxin loop II (nAChR inhibition site) is denoted by the blue arrow. (B) “Capture” of the lipid phosphatidylserine headgroup by the side chains of Glu-2, Asp-57, and Arg-58 from the NTII membrane-binding site. The toxin side chains and lipid molecule are shown in stick representation. The carbon, nitrogen, proton, oxygen, and phosphorus atoms of the toxin and lipid are colored in green, blue, white, red, and magenta, respectively. Intermolecular hydrogen bonds are indicated by dotted lines. The bilayer interface (average position of lipid headgroup phosphorus atoms) is depicted by the dashed line. (C) Electrostatic interaction of negatively charged phosphate and dipolar serine groups of DOPS with the NTII membrane-binding site, which is colored according to the surface electrostatic potential calculated using the DelPhi program (41).
