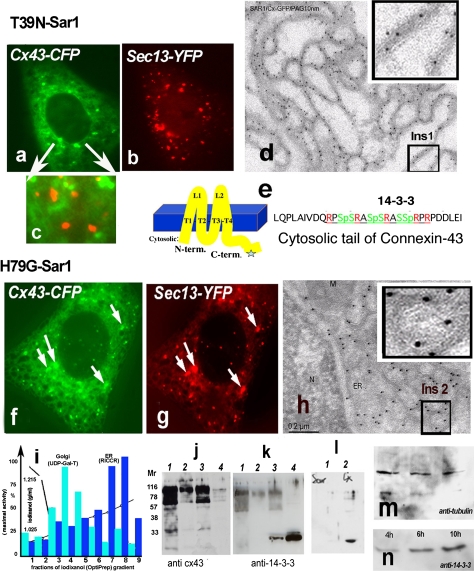
Fig. 2.
a–h Two distinct states of Cx43 in the ER in Vero cells expressing Sar1T39N or Sar1H79G. Only Sar1H79G accumulates Cx43 in ER exit sites (ERES) (h). This state correlates with accumulation of 14-3-3 molecules in the ER fraction (see k). a–c Cx43-GFP in cells co-transfected with Sar1T39N remains in the ER membranes, does not segregate into exit sites and does not co-localize with the COPII coat (c, overlay). d Immuno-EM analysis of ultrathin cryosections of Vero cells co-transfected with Cx43-GFP and Sar1T39N probed with anti-GFP antibodies and protein A-10 nm gold revealed that Cx43 immunoreactivity was distributed evenly along an extended ER network and not concentrated in the characteristically rounded ERES. A region of interest (Ins 1) is shown enlarged. e Cx43 transmembrane topology and acid sequence at the cytosolic C-terminus of Cx43 harboring serines in a context suitable for phosphorylation, 14-3-3 protein interaction and RPR/COPI binding motifs. f–g Sar1H79G induces colocalization of Cx43 with the Sec13 subunit of the COPII coat. h Tokuyasu cryo-sections of Vero cells co-transfected with Cx43-GFP and Sar1H79G. Immunogold staining with anti-GFP antibodies show ER cisternae and clearly detectable rounded ERES structures that are decorated with Cx43-GFP. A region of interest showing Cx43 concentrated in ERES is shown (Ins 2). Co-localization between Cx43 and the COPII subunit Sec13-YFP is increased in cells co-expressing Sar1H79G (f–g). i Subcellular fractionation of monolayer-grown Vero cells. ER and Golgi fractions were separated from the cytosol and PM fractions and tested for enzymatic activity: UDP-Gal-Transferase for the Golgi; Rotenon-Insensitive Cytochrome C-Reductase for the ER. Total protein concentrations in the fractions are indicated. j–k Proteins from combined ER fractions 7 and 8 (i) were resolved by SDS-PAGE and immunoblotted with anti-Cx43 and Pan-14-3-3 antibodies. Lanes are as follows: 1 ER fraction of cells transfected with Sar1T39N, 2 control Golgi fraction, 3 ER fraction of cells transfected with Sar1H79G, 4 cytosol. ER fractions (7–8 of the gradient, i) usually show upper oligomeric bands of Cx43 (j) with the Cx43 antibodies; these bands are absent in the cytosol fraction (j, lane 4) (k). Note that 14-3-3 accumulation is observed only in the ER fraction of cells transfected with Sar1H79G. 14-3-3 proteins are abundantly present in cytosol, used as a positive control. l Vero cells transfected with Sar1H79G alone do not show accumulation of 14-3-3 immunoreactivity in the ER (l, 1). Accumulation of 14-3-3 proteins in the ER fraction is seen only in cells co-transfected with Cx43 and Sar1H79G (l, 2). m–n Time-dependent accumulation of 14-3-3 proteins in the ER fraction of Vero cells co-transfected with Cx43 and Sar1H79G (4–10 h). The same membrane was reprobed with anti-tubulin as a loading control