Abstract
Aging is associated with profound changes in the structure and function of the heart. A fundamental understanding of these processes, using relevant animal models, is required for effective prevention and treatment of cardiovascular disease in the elderly. Here, we studied cardiac performance in 4- to 5-mo-old (young) and 24- to 26-mo-old (old) Fischer 344 male rats using the Millar pressure-volume (P-V) conductance catheter system. We evaluated systolic and diastolic function in vivo at different preloads, including preload recruitable stroke work (PRSW), maximal slope of the systolic pressure increment (+dP/dt), and its relation to end-diastolic volume (+dP/dt-EDV) as well as the time constant of left ventricular pressure decay, as an index of relaxation. The slope of the end-diastolic P-V relation (EDPVR), an index of left ventricular stiffness, was also calculated. Aging was associated with decrease in left ventricular systolic pressure, +dP/dt, maximal slope of the diastolic pressure decrement, +dP/dt-EDV, PRSW, ejection fraction, stroke volume, cardiac and stroke work indexes, and efficiency. In contrast, total peripheral resistance, left ventricular end-diastolic volume, left ventricular end-diastolic pressure, and EDPVR were greater in aging than in young animals. Taken together, these data suggest that advanced aging is characterized by decreased systolic performance accompanied by delayed relaxation and increased diastolic stiffness of the heart in male Fischer 344 rats. P-V analysis is a sensitive method to determine cardiac function in rats.
Keywords: systolic dysfunction, diastolic dysfunction
In the absence of obvious disease, the major anatomic changes seen in the aging human heart are primarily an increase in ventricular wall thickness, myocardial fibrosis, and valvular fibrocalcification, which lead to reduced ventricular compliance (26). In addition, loss of arterial elasticity produces a widening of arterial pulse pressure and reduction of the diastolic pressure that largely determines coronary artery perfusion. Aging also imposes a ceiling on maximal cardiac output (CO) that reflects both reduced maximal heart rate (HR) and the age-related prolongation of time required for both myocardial contraction and relaxation (25, 26). In a variety of mammals, including dogs and rodents, aging is also associated with hypertrophy, dilatation, and fibrosis of the left ventricle (LV) (1-3, 5-11, 21, 25, 37-39). A fundamental understanding of these processes, using relevant animal models, is required for more effective prevention and treatment of cardiovascular disease in the elderly. The Fischer 344 (F344) rat, developed by the National Institute on Aging, is an inbred rat model that is commonly used for aging studies. It has a longer lifespan, smaller body size, and lower spontaneous tumor rate than noninbred rats and resemble many features of aging humans. The F344 rat has served as a model to assess the mechanisms of age-related changes in the heart and their implications for the response of the heart to physiological and pathological challenges (1-3, 5-11, 21, 37-39).
The major limitation of the previously used invasive and noninvasive approaches to study the cardiac function in small animal models is that the hemodynamic parameters measured were largely dependent on loading conditions. Pressure-volume (P-V) analysis is a useful approach for examining intact chamber function independently of loading conditions. This analysis has been widely used in large animal studies and in humans (22-24, 27, 31, 36). Recent advances in the development and validation of miniature P-V catheters has made it possible to use this approach for studies in small animals (16, 18, 19, 29, 32, 41). Although P-V loop analysis is widely used in mice, the combined P-V conductance catheter for rats has been introduced only recently, and very limited normative data are available.
Here, we aimed to characterize the baseline cardiac performance and systolic and diastolic function at different preloads in aging F344 male rats using the Millar P-V conductance catheter system.
METHODS
This investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and was performed with the approval of the local Institutional Animal Care and Use Committee.
Animals
Male F344 rats were obtained from the National Institute on Aging at 3 (n = 12) and 24 mo of age (n = 20) and studied 1–2 mo later. Rats were housed two to three per cage, fed a standard laboratory diet and water ad libitum, and maintained on a 12:12-h light-dark cycle. During the observation period, ~50% of the aging and none of young control rats died, and autopsy revealed in most of the cases large dilated hearts and marked fluid accumulation in the chest cavity.
Hemodynamic measurements
Rats were anesthetized with thiopental sodium (60–80 mg/kg ip): aging animals with heart failure required ~15–20% less anesthetic than young rats. The animals were tracheotomized to facilitate breathing and placed on controlled heating pads. The core body temperature measured via a rectal probe was maintained at 37°C. A microtip P-V catheter (SPR-838, Millar Instruments; Houston, TX) was inserted into the right carotid artery and advanced into the LV under pressure control as described previously (32-34). Polyethylene (PE) catheters (PE-50) were inserted into the right femoral artery for measurement of mean arterial pressure (MAP) and the right femoral vein for fluid administration. After stabilization for 20 min, the signals were continuously recorded at sampling rate of 1,000/s using an ARIA P-V conductance system (Millar Instruments) coupled to a PowerLab/4SP A/D converter (AD Instruments; Mountain View, CA) and a personal computer.
HR, maximal LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), MAP, maximal slope of systolic pressure increment (+dP/dt) and diastolic pressure decrement (−dP/dt), time constant of LV pressure decay (τ), ejection fraction (EF), stroke volume (SV), end-diastolic volume (EDV), CO, and stroke work (SW) were computed using a cardiac P-V analysis program (PVAN3.2, Millar Instruments) as described previously (13, 32). SV and CO were calculated and corrected according to in vitro and in vivo volume calibrations using PVAN3.2 (32). CO and SW were normalized to body weight [cardiac index (CI) and SW index (SWI)]. The total peripheral resistance index (TPRI) was calculated by the following equation: TPRI = MAP/CI.
LV P-V relations were assessed by transiently compressing the inferior vena cava. Indexes of contractility and LV stiffness {preload recruitable stroke work (PRSW), +dP/dt-end-diastolic volume relation (+dP/dt-EDV), slope of end-systolic and end-diastolic P-V relations [ESPVR (Emax) and EDPVR]} as well as the efficiency of LV work [SW/P-V area (PVA)] were calculated using PVAN3.2.
The volume calibration of the conductance system was performed as described previously (32, 31). Briefly, nine cylindrical holes in a block 1 cm deep and with known diameter ranging from 2 to 11 mm were filled with fresh heparinized whole rat blood. In this calibration, the linear volume-conductance regression of the absolute volume in each cylinder versus the raw signal acquired by the conductance catheter was used as the volume calibration formula (29, 32, 41). At the end of each experiment, 50 μl of 15% saline were injected intravenously, and from the shift of P-V relations parallel conductance volume (Vp) was calculated by PVAN 3.2 and used for correction for the cardiac mass volume as described previously (32, 41).
Cardiac remodeling
After the hemodynamic measurements, the animals were killed by exsanguination (transsection of the inferior vena cava). The heart was removed, and the LV and right ventricle were dissected and weighed (the septum was included in the LV weight). The weight of each ventricle was then normalized to body weight. The lungs were also removed and weighed. Wet lung weights were normalized to body weight, and the ratio was used as an index of pulmonary congestion (28, 33).
Statistical analysis
An unpaired two-sided Student’s t-test was used to compare tissue weights and hemodynamic parameters of aging and young rats. Values of P < 0.05 were considered statistically significant.
RESULTS
Cardiac remodeling
Aging was associated with a significant increase in the weight of the LV but not the right ventricle: LV weights in young (4–5 mo old, n = 8) and aging (24–26 mo old, n = 10) rats normalized to body weight were 1.7 ± 0.05 and 2.4 ± 0.05 mg/g, respectively (P < 0.05), whereas the normalized right ventricular weights were 0.4 ± 0.01 and 0.4 ± 0.03 mg/g, respectively. In addition, wet lung weight normalized to body weight was also significantly increased in aging animals compared with the young controls (5.9 ± 0.3 vs. 4.5 ± 0.26 mg/g, respectively, P < 0.05), indicating marked pulmonary congestion secondary to cardiac decompensation.
Cardiac function
Figure 1 shows typical P-V loops (top) and the pressure signal and dP/dt (bottom) obtained from young and aging animals. The shift of the P-V loops to the right and the decreased amplitude and +dP/dt in aging rats indicates a decrease in cardiac contractility. As shown in Table 1, aging was associated with decreased MAP, LVSP, +dP/dt, −dP/dt, EF, SV, CI, and SWI. In contrast, LVEDP, τ, and EDV were increased in aging animals, indicating diastolic dysfunction. TPRI was also increased in aging rats. There was a slight (11%) but not significant decrease in the HR of aging animals (Table 1).
Fig. 1.
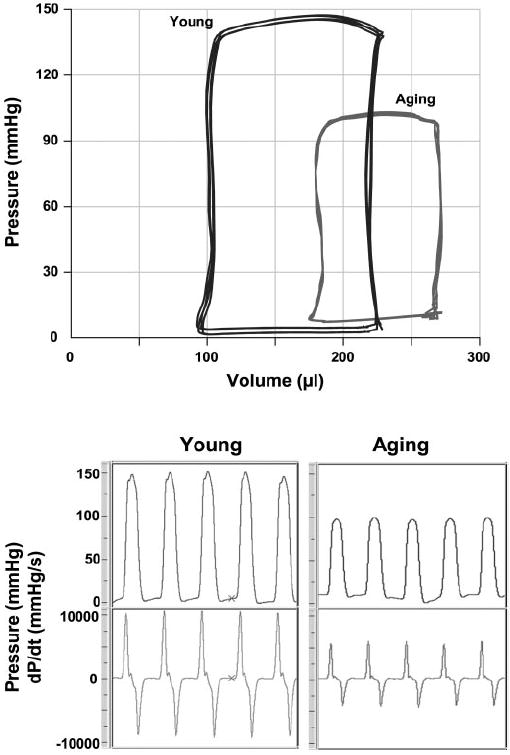
Representative pressure-volume (P-V) loops (top) and pressure signals and dP/dt (bottom) obtained with the Millar P-V conductance catheter system from aging and young rats. The rightward shift of the P-V loop and decrease of the amplitude of the pressure signal and +dP/dt indicates decreased contractility in aging rats compared with young controls.
Table 1.
Hemodynamic parameters in young and aging rats measured by the Millar pressure-volume conductance catheter system
| Young | Aging | |
|---|---|---|
| HR, beats/min | 403.1±11.6 | 359.2±20.2 |
| MAP, mmHg | 109.2±3.1 | 83.6±2.0* |
| LVSP, mmHg | 133.1±6.1 | 98.4±2.3* |
| LVEDP, mmHg | 3.8±0.9 | 9.1±0.6* |
| CI, ml·min−1·100 g−1 | 13.7±1.4 | 7.6±0.7* |
| EF, % | 72.7±7.0 | 41.6±5.4* |
| SWI, mmHg·ml·100 g−1 | 4,612±590 | 1,850±198* |
| +dP/dt, mmHg/s | 9,327±556 | 5,975±204* |
| −dP/dt, mmHg/s | 7,721±546 | 4,580±298* |
| τ (Weiss), ms | 7.8±0.4 | 11.2±0.6* |
| τ (Glantz), ms | 11.5±0.4 | 15.9±0.2* |
| TPRI, mmHg·ml−1·min−1·100 g−1 | 8.2±0.5 | 11.6±1.1* |
| SV, μl | 124.3±8.8 | 87.9±7.6* |
| EDV, μl | 199.2±14.4 | 263.2±17.5* |
| Emax, mmHg/μl | 2.6±0.2 | 1.2±0.2* |
| EDPVR slope, mmHg/μl | 0.021±0.003 | 0.04±0.004* |
| Efficiency, % | 66.2±1.9 | 43.4±2.2* |
Values are means ± SE; n = 8–10 experiments. HR, heart rate; MAP, mean arterial pressure; LVSP, left ventricular (LV) systolic pressure; LVEDP, LV end-diastolic pressure; CI, cardiac index; EF, ejection fraction; SWI, stroke work index, +dP/dt and −dP/dt, maximal slope of the systolic pressure increment and diastolic pressure decrement, respectively; τ, time constant of LV pressure decay; SV, stroke volume; EDV, end-diastolic volume; Emax, maximum elastance; EDPVR, end-diastolic pressure-volume relation.
P < 0.05 vs. young.
Figure 2 displays typical P-V loops obtained after inferior vena cava occlusions in aging (bottom) and young (top) rats. Overall results of EDPVR and ESPVR (Emax) are depicted in Table 1. As shown in Fig. 2 and Table 1, ESPVR was steeper in young animals, suggesting decreased systolic performance in aging rats. In contrast, EDPVR was increased in aging rats, indicating increased end-diastolic stiffness. In addition to the above parameters, P-V loops recorded at different preloads can be used to derive other useful systolic function indexes that may be less influenced by loading conditions and cardiac mass, such as PRSW and +dP/dt-EDV (24, 27, 36) (Fig. 3).
Fig. 2.
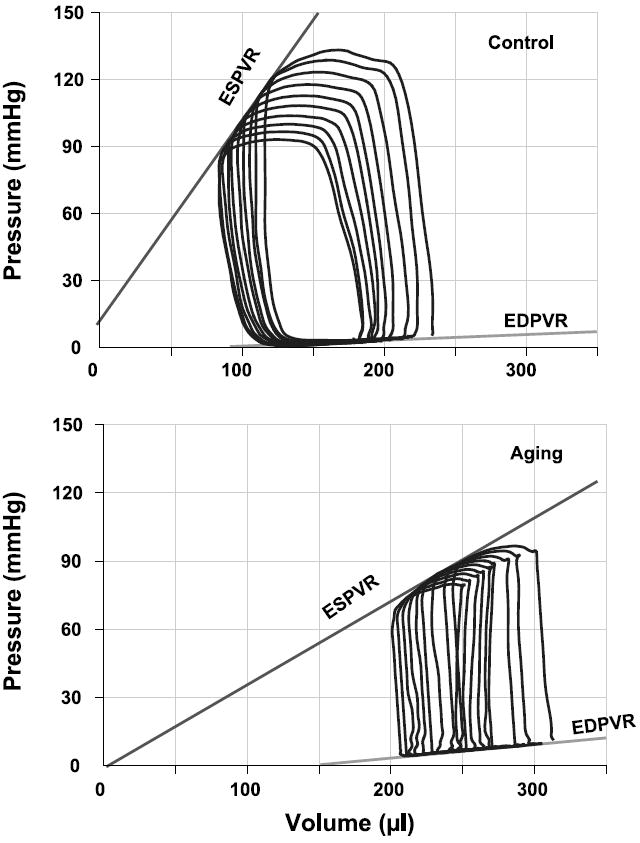
Representative P-V loops obtained with a P-V conductance catheter system at different preloads, showing differences in the end-systolic P-V relation (ESPVR) and end-diastolic P-V relation (EDPVR) between young (top) and aging rats (bottom). The less steep ESPVR and increased EDPVR in aging animals indicate decreased contractile function and increased diastolic stiffness.
Fig. 3.
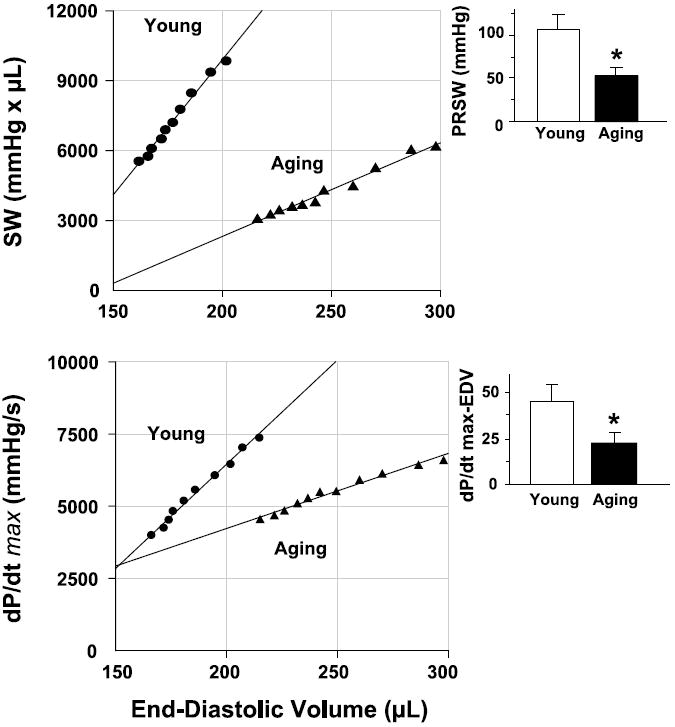
Relation between stroke work (SW) and end-diastolic volume (EDV; top) and between +dP/dt and EDV (bottom) for a young (●) and an aging rats (▲). PRSW, preload recruitable SW. Insets: overall slope values as means ± SE of 8–10 experiments in each group. *P < 0.05 vs. young rats. Note that for both relationships, slope values are higher in young than in aging rats, suggesting that systolic performance is decreased in aging rats.
PRSW represents the slope of the relation between SW and EDV. It has been described as independent of chamber size and mass, and it is sensitive to chamber contractile function (22, 23). Figure 3, top, shows PRSW in a young and an aging animal. The slope is steeper in the young rat than in the aging rat, indicating decreased systolic performance in the aging animal. The overall PRSW was significantly higher in young rats (Fig. 3, top, inset).
We also determined the relation between +dP/dt and EDV. +dP/dt is a classic parameter that is sensitive to changes in contractility but dependent on changes in preload. Analysis of +dP/dt-EDV allowed us to compare +dP/dt of aging and young rats at a given EDV. Figure 3, bottom, shows that the slope of this relation was steeper in young rats, indicating a decreased contractility in aging.
Efficiency of LV work was also decreased in aging rats compared with young rats (Table 1).
DISCUSSION
To our knowledge, this is the first study to characterize the systolic and diastolic function in an advanced aging-associated heart failure model using a P-V conductance catheter system. Here, we show that advanced aging is characterized by decreased systolic performance accompanied by delayed relaxation and increased diastolic stiffness of the heart.
We found that aging was associated with decreased +dP/dt and EF (Table 1). Although +dP/dt has been widely used as a cardiac contractile parameter, it is well recognized that it is load dependent, especially on changes in preload (22-24, 27). EF is also known to be influenced by both preload and afterload and, therefore, cannot reliably be used to assess the contractile function in the models where both preload and afterload are altered.
Historically, ESPVR (Emax) was proposed as a fairly load-insensitive index of contractility. ESPVR was decreased in aging rats. However, because this relation can be altered not only by changes in inotropic state but also by changes in chamber geometry and other diastolic factors, we also calculated other parameters (22, 23, 31).
PRSW, the linear relation between SW and EDV, may be sensitive to changes in systolic function. It has advantage of being stable in the hearts of different sizes. Although it integrates data from the entire cardiac cycle, it is influenced most of all by systole (22, 23). PRSW was also decreased in aging rats compared with young controls.
Previous investigations (24, 27) have demonstrated that +dP/dt-EDV, another P-V-derived index, represents a sensitive but less load-dependent parameter of chamber contractility. In previous studies, the slope of this relationship increased and shifted leftward with increased contractility, and it decreased and shifted rightward with depressed contractility. We found that this index was also depressed in aging animals compared with young controls.
Our baseline hemodynamic data obtained with the P-V system are in a good agreement with the results of Capasso et al. (9). In that study, F344 rats were anesthetized with chloral hydrate (300 mg/kg ip) followed by measurements of ventricular pressure-derived parameters and ascending aortic flow. From 20 mo of age, there was an elevation in LVEDP, a significant decrease in +dP/dt and −dP/dt, a reduction of SV and CO, and an increase in total peripheral resistance. At 29 mo of age, EF, +dP/dt, −dP/dt, SV, CO, and HR were reduced by 59, 38, 48, 27, 40, and 17%, respectively, whereas LVEDP and total peripheral resistance increased by 275 and 59%, respectively (9). Similarly, in our study in barbiturate-anesthetized F344 male rats using P-V analysis, at 26 mo of age, EF, +dP/dt, −dP/dt, SV, CI, and HR were reduced by 43, 36, 41, 29, 44, and 11%, respectively, whereas LVEDP and total peripheral resistance increased by 139 and 41%, respectively (Table 1). In addition to the baseline parameters of contractility, we showed that all load-independent indexes of LV contractility (see above and also Table 1 and Figs. 2 and 3) demonstrate decreased contractile pump function in aging rats. The indexes of LV failure were accompanied by alterations in ventricular size and shape consisting of increases in the transverse and longitudinal chamber diameters and an abnormal expansion in diastolic and systolic ventricular chamber volumes. Consistent with the expansion of the diastolic chamber volume in the previous report (9), we also observe an increase in EDV in aging F344 rats using the P-V system (Table 1). A similar age-associated decline in cardiac function was also reported in mice using the P-V technique (41).
In a more recent study, Boluyt et al. (5) confirmed both systolic and diastolic dysfunction in isoflurane-anesthetized 30-mo-old female F344 rats using echocardiographic assessment. However, in the latter study, the reduction of systolic performance was less pronounced (EF and fractional shortening were decreased by 9 and 25%, respectively) than it was demonstated by Capasso et al. (9) and by the present investigation (see above). The reason for the less depressed systolic function found by Boluyt et al. (5) can be the protection against development of the cardiac dysfunction and heart failure in female F344 rats compared with male rats used in the previous (9) and our present studies.
One can also argue that aged rats are more sensitive to the cardiovascular depressant effects of barbiturate anesthesia than younger animals, and this could have a significant impact on the magnitude of changes noted in the aged versus younger animals. Although there is no perfect pharmacological agent available for anesthesia in the elderly, dozens of clinical studies have arrived to the same conclusion that no significant difference in outcome can be attributed solely to use of any specific anesthetic agent (4). Barbiturates have been a standard element of anesthesia practice both in the young and elderly patients for more that 50 years. Individual variability in response to thiopental is well known. In elderly patients, an age-related decrease in the initial distribution volume or differences in clearance to the rapidly equilibrating compartments were found (20). Compared with younger patients, geriatric patients require a 20–30% lesser induction dose (12). On the basis of the latter study, we also used a reduced dose of the drug in our aging rats (see METHODS). The predominant cardiovascular effect of thiopental is venodilation, followed by pooling of blood in the periphery (14). Myocardial contractility may be depressed, but not to the extent seen after volatile anesthetics (17). Importantly, barbiturates do not sensitize the heart to cathecholamines. Systemic vascular resistance usually remains unchanged, and no arrhythmia occurs. We have successfully used barbiturates for anesthesia in various rat models of shock, myocardial infarction, and heart failure and reported comparable, if not better, hemodynamic parameters than using inhalation agents (15, 33, 34). In a previous study (35) using 22-mo-old aging Wistar rats, we reported impaired endothelial nitric oxide-mediated dilation of isolated aortic rings without any depression of systolic cardiac function using the same anesthesia protocol. Thus it is unlikely that the increased sensitivity of aging animals to thiopental could have a significant contribution to the hemodynamic changes observed in the present study.
Impaired ventricular relaxation and increased end-diastolic stiffness were also observed in aging animals, as reflected by the decreased −dP/dt, prolonged τ, and increased LVEDP and EDPVR. Relaxation, an active process, depends mostly on calcium uptake by the sarcoplasmic reticulum during diastole, and under normal conditions usually takes place during the first third of diastole. End-diastolic stiffness is predominantly affected by alterations in myocardial structural components; however, it may also be influenced by various other factors. We observed both delayed relaxation and increased stiffness in aging rats. Consistent with diastolic dysfunction, LVEDP was also increased in aging animals.
Potential limitations of the study
P-V loop analysis, which is widely used in mice, has become a prerequisite for the assessment of LV function because it is the only technique that allows measuring the LV performance independently from loading conditions. The combined pressure-conductance probe for rats has been introduced only recently, and limited normative data are available. The proper measurement of absolute volumes is the most vulnerable part of the P-V technique, especially in a situation that requires the comparison of groups with markedly different ventricular sizes. In these conditions, problems could arise from the potential limitation in the construction of the probe, which has only two pairs of conductance electrodes at the fixed distance from each other. Nevertheless, as our results demonstrate, the baseline P-V data are very similar to the earlier data obtained by using flow measurements in the same aging-associated heart failure model (9). Importantly, our baseline SV data in control young adult rats are very similar to the data recently obtained by MRI (124.3 ± 8.8 vs. 126.8 ± 8 μl) (30), also suggesting that in most earlier studies the rat SV and CO were markedly overestimated.
Because aging is also associated with vascular dysfunction and remodeling, it is conceivable that the impairment of vascular function and the aging-associated decrease in cardiac contractility and heart failure are interrelated: an impairment of endothelial function may lead to global or regional myocardial ischemia, which may secondarily impair cardiac performance. Therefore, myocardial aging in this model should rather be interpreted as a time-dependent biological process that interacts with ischemic heart disease and other pathological conditions, which together define the phenotype.
In conclusion, our data demonstrate decreased systolic performance accompanied by delayed relaxation and increased diastolic stiffness of the heart in the advanced model of aging-associated heart failure using the Millar P-V conductance catheter system. The P-V methodology could be a very useful approach for the assessment of LV function in various pathophysiological conditions associated with cardiac dysfunction and/or heart failure in rats, and, furthermore, it could be successfully applied to evaluate potential pharmacotherapies against these conditions.
Acknowledgments
Authors are indebted to Millar Instruments for the excellent technical support during the course of the study.
GRANTS This study was supported by National Institute on Aging Grants 1R03-AG-21206-01 and R0159266.
References
- 1.Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Physiol Heart Circ Physiol. 1994;267:H1062–H1073. doi: 10.1152/ajpheart.1994.267.3.H1062. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Puntillo E, Nikitin P, Olivetti G, Capasso JM, Sonnenblick EH. Effects of age on mechanical and structural properties of myocardium of Fischer 344 rats. Am J Physiol Heart Circ Physiol. 1989;256:H1440–H1449. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- 4.Bode RH, Jr, Lewis KP, Zarich SW, Pierce ET, Roberts M, Kowalchuk GJ, Satwicz PR, Gibbons GW, Hunter JA, Espanola CC. Cardiac outcome after peripheral vascular surgery. Comparison of general and regional anesthesia. Anesthesiology. 1996;84:3–13. doi: 10.1097/00000542-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 6.Boluyt MO, Devor ST, Opiteck JA, White TP. Age effects on the adaptive response of the female rat heart following aortic constriction. J Gerontol A Biol Sci Med Sci. 2000;55:B307–B314. doi: 10.1093/gerona/55.6.b307. [DOI] [PubMed] [Google Scholar]
- 7.Boluyt MO, Opiteck JA, Esser KA, White TP. Cardiac adaptations to aortic constriction in adult and aged rats. Am J Physiol Heart Circ Physiol. 1989;257:H643–H648. doi: 10.1152/ajpheart.1989.257.2.H643. [DOI] [PubMed] [Google Scholar]
- 8.Capasso JM, Fitzpatrick D, Anversa P. Cellular mechanisms of ventricular failure: myocyte kinetics and geometry with age. Am J Physiol Heart Circ Physiol. 1992;262:H1770–H1781. doi: 10.1152/ajpheart.1992.262.6.H1770. [DOI] [PubMed] [Google Scholar]
- 9.Capasso JM, Palackal T, Olivetti G, Anversa P. Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Am J Physiol Heart Circ Physiol. 1990;259:H1086–H1096. doi: 10.1152/ajpheart.1990.259.4.H1086. [DOI] [PubMed] [Google Scholar]
- 10.Capasso JM, Puntillo E, Olivetti G, Anversa P. Differences in load dependence of relaxation between the left and right ventricular myocardium as a function of age in rats. Circ Res. 1989;65:1499–1507. doi: 10.1161/01.res.65.6.1499. [DOI] [PubMed] [Google Scholar]
- 11.Cheng W, Reiss K, Li P, Chun MJ, Kajstura J, Olivetti G, Anversa P. Aging does not affect the activation of the myocyte insulin-like growth factor-1 autocrine system after infarction and ventricular failure in Fischer 344 rats. Circ Res. 1996;78:536–546. doi: 10.1161/01.res.78.4.536. [DOI] [PubMed] [Google Scholar]
- 12.Christensen JH, Andreasen F. Individual variation in response to thiopental. Acta Anaesthesiol Scand. 1978;22:303–313. doi: 10.1111/j.1399-6576.1978.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 13.Cingolani OH, Yang XP, Cavasin MA, Carretero OA. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertensive rats. Hypertension. 2003;41:249–254. doi: 10.1161/01.hyp.0000052832.96564.0b. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein JW, Hamilton WK, McCammond JM. The effect of thiopental on peripheral venous tone. Anesthesiology. 1961;22:525–528. doi: 10.1097/00000542-196107000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Evgenov OV, Pacher P, Williams W, Evgenov NV, Mabley JG, Cicila J, Siko ZB, Salzman AL, Szabo C. Parenteral administration of glipizide sodium salt, an inhibitor of adenosine triphosphate-sensitive potassium channels, prolongs short-term survival after severe controlled hemorrhage in rats. Crit Care Med. 2003;31:2429–2436. doi: 10.1097/01.CCM.0000089639.84344.A7. [DOI] [PubMed] [Google Scholar]
- 16.Feldman MD, Erikson JM, Mao Y, Korcarz CE, Lang RM, Freeman GL. Validation of a mouse conductance system to determine LV volume: comparison to echocardiography and crystals. Am J Physiol Heart Circ Physiol. 2000;279:H1698–H1707. doi: 10.1152/ajpheart.2000.279.4.H1698. [DOI] [PubMed] [Google Scholar]
- 17.Frankl WS, Poole-Wilson PA. Effects of thiopental on tension development, action potential, and exchange of calcium and potassium in rabbit ventricular myocardium. J Cardiovasc Pharmacol. 1981;3:554–565. doi: 10.1097/00005344-198105000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Georgakopoulos D, Kass D. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol. 2001;534:535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol Heart Circ Physiol. 1998;274:H1416–H1422. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- 20.Homer TD, Stanski DR. The effect of increasing age on thiopental disposition and anesthetic requirement. Anesthesiology. 1985;62:714–724. doi: 10.1097/00000542-198506000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol Heart Circ Physiol. 1996;271:H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- 22.Kass DA. Clinical ventricular pathophysiology: a pressure-volume view. In: Warltier DC, editor. Ventricular Function. Baltimore, MD: Williams & Wilkins; 1995. pp. 131–151. [Google Scholar]
- 23.Kass DA. Myocardial mechanics. In: Poole-Wilson PA, Colucci WS, Massie BM, Chatterjee K, Coast AJS, editors. Heart Failure Scientific Principles and Clinical Practice. New York: Churchill Livingstone; 1997. pp. 87–108. [Google Scholar]
- 24.Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- 25.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 27.Little WC. The left ventricular dP/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res. 1985;56:808–815. doi: 10.1161/01.res.56.6.808. [DOI] [PubMed] [Google Scholar]
- 28.Maxey TS, Enelow RI, Gaston B, Kron IL, Laubach VE, Doctor A. Tumor necrosis factor-alpha from resident lung cells is a key initiating factor in pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2004;127:541–547. doi: 10.1016/j.jtcvs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, Marban E. Transgenic mouse model of stunned myocardium. Science. 2000;287:488–491. doi: 10.1126/science.287.5452.488. [DOI] [PubMed] [Google Scholar]
- 30.Nahrendorf M, Hiller KH, Greiser A, Kohler S, Neuberger T, Hu K, Waller C, Albrecht M, Neubauer S, Haase A, Ertl G, Bauer WR. Chronic coronary artery stenosis induces impaired function of remote myocardium: MRI and spectroscopy study in rat. Am J Physiol Heart Circ Physiol. 2003;285:H2712–H2721. doi: 10.1152/ajpheart.00233.2003. [DOI] [PubMed] [Google Scholar]
- 31.Nakano K, Sugawara M, Ishihara K, Kanazawa S, Corin WJ, Denslow S, Biederman RW, Carabello BA. Myocardial stiffness derived from end-systolic wall stress and logarithm of reciprocal of wall thickness. Contractility index independent of ventricular size. Circulation. 1990;82:1352–1361. doi: 10.1161/01.cir.82.4.1352. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 33.Pacher P, Liaudet L, Mabley J, Komjati K, Szabo C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- 34.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 35.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagawa K, Maughan L, Suga H, Sunagava K. Cardiac Contraction and Pressure-Volume Relationship. New York: Oxford University Press; 1988. [Google Scholar]
- 37.Thomas DP, Cotter TA, Li X, McCormick RJ, Gosselin LE. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur J Appl Physiol. 2001;85:164–169. doi: 10.1007/s004210100447. [DOI] [PubMed] [Google Scholar]
- 38.Thomas DP, McCormick RJ, Zimmerman SD, Vadlamudi RK, Gosselin LE. Aging- and training-induced alterations in collagen characteristics of rat left ventricle and papillary muscle. Am J Physiol Heart Circ Physiol. 1992;263:H778–H783. doi: 10.1152/ajpheart.1992.263.3.H778. [DOI] [PubMed] [Google Scholar]
- 39.Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol. 2000;89:1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- 40.Wada DR, Bjorkman S, Ebling WF, Harashima H, Harapat SR, Stanski DR. Computer simulation of the effects of alterations in blood flows and body composition on thiopental pharmacokinetics in humans. Anesthesiology. 1997;87:884–899. doi: 10.1097/00000542-199710000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol Heart Circ Physiol. 1999;277:H1906–H1913. doi: 10.1152/ajpheart.1999.277.5.H1906. [DOI] [PubMed] [Google Scholar]


