Abstract
Shovel shape of upper incisors is a common characteristic in Asian and Native American populations but is rare or absent in African and European populations. Like other common dental traits, genetic polymorphisms involved in the tooth shoveling have not yet been clarified. In ectodysplasin A receptor (EDAR), where dysfunctional mutations cause hypohidrotic ectodermal dysplasia, there is a nonsynonymous-derived variant, 1540C (rs3827760), that has a geographic distribution similar to that of the tooth shoveling. This allele has been recently reported to be associated with Asian-specific hair thickness. We aimed to clarify whether EDAR 1540C is also associated with dental morphology. For this purpose, we measured crown diameters and tooth-shoveling grades and analyzed the correlations between the dental traits and EDAR genotypes in two Japanese populations, inhabitants around Tokyo and in Sakishima Islands. The number of EDAR 1540C alleles in an individual was strongly correlated with the tooth-shoveling grade (p = 7.7 × 10−10). The effect of the allele was additive and explained 18.9% of the total variance in the shoveling grade, which corresponds to about one-fourth of the heritability of the trait reported previously. For data reduction of individual-level metric data, we applied a principal-component analysis, which yielded PC1-4, corresponding to four patterns of tooth size; this result implies that multiple factors are involved in dental morphology. The 1540C allele also significantly affected PC1 (p = 4.9 × 10−3), which denotes overall tooth size, and PC2 (p = 2.6 × 10−3), which denotes the ratio of mesiodistal diameter to buccolingual diameter.
Main Text
Teeth display variations among individuals in the size and the shape of cusps, ridges, grooves, and roots. In addition, certain dental characteristics are predominant in certain populations.1,2 In Asian and Asia-derived populations, dental variations have often been described as “Sinodonty” and “Sundadonty.” Sinodonty, common among East Asian and Native American populations, is a combination of dental characteristics that relatively often include upper first and second incisors (UI1 and UI2) that are shovel-shaped and not aligned with the other teeth, upper first premolars (UP1) with one root, and lower first molars (LM1) with three roots.3 In particular, the prevalence of tooth shoveling shows marked regional differences, which is frequent in Asia, with a south-to-north increasing cline, but rare or absent in Africa and Europe.4,5 Tooth shoveling has thus received much attention from anthropologists as an indicator of relationships among populations. The well-known contrast between ancient and modern Asians is that remains of the Jomon people, who were the indigenous Japanese 12,000–2,000 years ago, have a smaller tooth size and a lower tooth-shoveling grade than modern Japanese.1,6–8 In addition, the continuity of shovel-shaped incisors between Homo erectus and modern humans in East Asia was a rationale for the multiregional evolution theory of modern humans,9,10 although this theory is not generally supported at present.
Characteristics of dental morphology are thought to be determined predominantly by genetic factors.5 However, genetic polymorphisms associated with common dental traits have not yet been elucidated. For tooth shoveling, high concordance between monozygotic twins has been observed.11,12 On the basis of intrafamilial correlations, three different studies have reported that the heritability of tooth shoveling was around 0.75 in Asian and Native American groups.5,11,13
The recent availability of large-scale data on human genome diversity has enabled us to screen genes highly differentiated between populations; these make reasonable candidate genes for population-specific phenotypes. One nonsynonymous-derived variant predominant in East Asian populations but absent in populations of African and European origins is present on rs3827760, a single-nucleotide polymorphism (SNP) in the ectodysplasin A receptor gene (EDAR [MIM 604095]), which is also called EDAR T1540C according to nucleotides or V370A according to amino acids.14–16 In humans, dysfunctional mutations in EDAR as well as the ectodysplasin A gene (EDA [MIM 300451]) are known to be responsible for hypohidrotic ectodermal dysplasia [MIM 129490, 224900, and 305100], a genetic disorder causing abnormal morphogenesis of teeth, hair, and sweat glands.17 Mouse and fish studies have also indicated that the Eda-Edar pathway regulates the development of organs, especially those of ectodermal origin such as hair, glands, nails, teeth, and scales.18–24 Therefore, EDAR T1540C can be considered a strong candidate for determining differences between human populations in visible traits of ectodermal appendices, and it has recently been reported that the EDAR 1540C allele is associated with Asian-specific hair thickness.25,26
The aim of this study is to clarify whether the nonsynonymous polymorphism in EDAR is also associated with human dental morphology. For this purpose, plaster casts of permanent dentition (Figure 1A) were obtained from 102 individuals at Showa University Dental Hospital, Tokyo, and from 100 individuals in Sakishima Islands (Miyako and Ishigaki), the southernmost Ryukyu Islands (Figure 2).27,28 The Sakishima subjects were selected as individuals who had no ancestor derived from other regions for at least three generations. We also collected a blood, buccal mucosa, or saliva specimen from each individual to prepare a DNA sample. DNA was extracted via standard methods. All the subjects gave informed consent to participate in the study. This study was conducted under the approval of ethical committees in Showa University, University of the Ryukyus, Tokai University, and the University of Tokyo.
Figure 1.
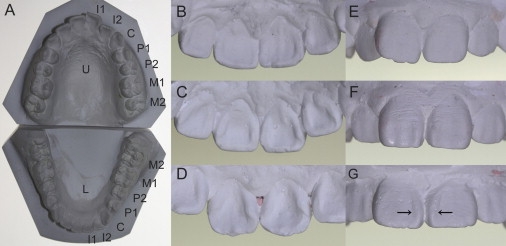
Evaluation of the Dental Morphology
(A) Plaster casts of upper and lower teeth. The following abbreviations are used: U, upper; L, lower; I, incisor; C, canine; P, premolar; and M, molar.
(B–D) Variation in shoveling, which refers to the development of marginal ridges on the lingual surfaces of UI1. (B) shows grade 1, (C) shows grade 3, and (D) shows grade 5.
(E–G) Variation in double shoveling, which refers to the development of marginal ridges on the labial surfaces of UI1 (E) grade 1, (F) grade 3, and (G) grade 5. Arrows indicate pronounced mesial marginal ridges.
Figure 2.
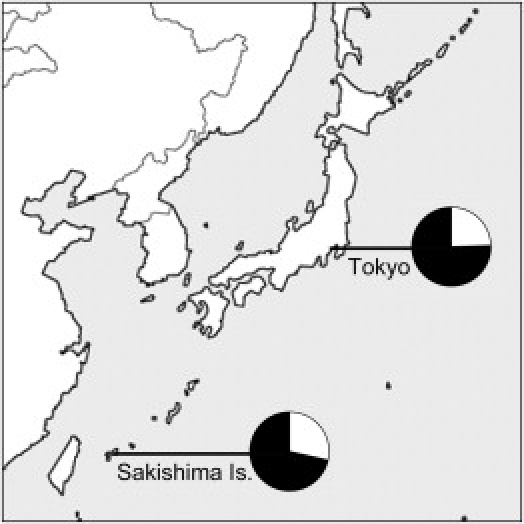
Locations and Allele Frequencies
Pie charts indicate the allele frequencies for EDAR 1540T (white) and 1540C (black).
Buccolingual and mesiodistal crown diameters of all the teeth except the third molars (M3) were measured with a sliding caliper. Shoveling of UI1 was graded on a scale of 0 to 7, according to the Arizona State University dental anthropology system (Figures 1B–1D).29 In addition, double shoveling, which refers to the development of mesial and distal marginal ridges on the labial surfaces of UI1, was graded on a scale of 0–6 (Figures 1E–1G). Measurements by multiple observers indicated an acceptably small observer error. The double-shoveling grade in females was significantly greater than that in males, and the same tendency was observed with the shoveling grade (Table S1, available online). Neither shoveling nor double-shoveling grades differed significantly between the Tokyo and Sakishima samples. Metric measurements showed sexual dimorphism, most markedly in the canines. A significant regional difference was observed for some teeth, especially in females, perhaps because of the larger sample size for females than for males. Interestingly, the Sakishima females exhibited a larger mesiodistal diameter of canines than did the Tokyo females.
To reduce the metric data to a small number of dimensions, we applied a principal-component analysis (PCA) with the data for 751 Japanese individuals (375 males and 376 females) from Honshu, Okinawa, and the Sakihima Islands.27,28,30 The procedure is as follows: the metric dental data, from which missing values were removed, were standardized for each sex. Then, merging the standardized data of both sexes, we created a correlation-coefficient matrix. PCA was performed with the matrix, and the eigenvalue and eigenvectors in each PC were obtained. Finally, we calculated PC scores for the 202 individuals with the DNA sample, where missing values were considered as 0 (i.e., the average after the standardization of data). The eigenvalue of the first PC (PC1) was 12.86, which occupied 45.9% (Table S2). From PC1 to PC4, the eigenvalue was larger than 1. This indicates that the first four PCs effectively represent the metric data. Figure 3 shows the eigenvectors in the PCs, demonstrating what each PC axis denotes: PC1, the overall tooth size; PC2, buccolingual versus mesiodistal diameter balance; PC3, anterior versus posterior size balance; and PC4, molar versus premolar size balance. In a comparison between PC scores for the Tokyo and Sakishima samples, we found that the Sakishima females had a higher PC1 score than the Tokyo females (Table S3). The shoveling and double-shoveling grades were strongly correlated (Table 1). The shoveling grade was strongly correlated not only with mesiodistal diameter of UI1 but also with PC1 and PC2. The double-shoveling grade was highly associated with PC3.
Figure 3.
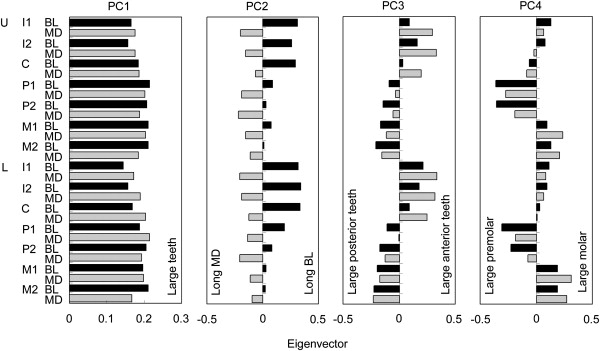
Eigenvectors in Principal Components
The following abbreviations are used: U, upper; L, lower; I, incisor; C, canine; P, premolar; M, molar; BL, buccolingual diameter; and MD, mesiodistal diameter.
Table 1.
Spearman's Rank Correlation Coefficients
| SH | DSH | UI1MD | PC1 | PC2 | PC3 | PC4 | C1487T | T1540C | |
|---|---|---|---|---|---|---|---|---|---|
| SH | 0.40 | 0.36 | 0.28 | −0.30 | 0.15 | −0.01 | 0.36 | 0.42 | |
| DSH | 9.0E-09 | 0.19 | 0.18 | −0.09 | 0.26 | 0.09 | 0.34 | 0.39 | |
| UI1MD | 1.9E-07 | 7.4E-03 | 0.60 | −0.40 | 0.31 | 0.09 | 0.17 | 0.20 | |
| PC1 | 6.0E-05 | 1.3E-02 | 1.1E-20 | −0.03 | −0.06 | −0.09 | 0.15 | 0.20 | |
| PC2 | 2.3E-05 | 1.8E-01 | 7.2E-09 | 6.6E-01 | −0.01 | −0.03 | −0.21 | −0.21 | |
| PC3 | 3.9E-02 | 1.6E-04 | 7.3E-06 | 3.9E-01 | 8.5E-01 | 0.08 | 0.05 | 0.07 | |
| PC4 | 9.4E-01 | 1.9E-01 | 1.9E-01 | 2.1E-01 | 6.2E-01 | 2.7E-01 | 0.13 | 0.14 | |
| EDAR C1487T | 1.8E-07 | 7.4E-07 | 1.7E-02 | 3.0E-02 | 3.2E-03 | 4.7E-01 | 6.5E-02 | 0.85 | |
| EDAR T1540C | 7.7E-10 | 1.2E-08 | 5.4E-03 | 4.9E-03 | 2.6E-03 | 3.2E-01 | 4.6E-02 | 1.0E-57 |
Spearman's Rho values are shown in the upper-right portion of the matrix, and p values are in the lower-left. The following abbreviations are used: SH, shoveling grade; DSH, double-shoveling grade; UI1, upper first incisor; and MD, mesiodistal diameter. p values less than 0.01 are shown in bold.
Genotyping for the EDAR T1540C polymorphism (rs3827760) was performed with the polymerase chain reaction (PCR)-direct sequencing method.26 In this sequencing, we also genotyped a neighboring synonymous polymorphism in the EDAR coding region, C1487T (rs12623957). The frequencies of the EDAR 1540C allele were 0.755 and 0.715 in the Tokyo and Sakishima samples, respectively (Table S4), which were not significantly different. The genotype for EDAR T1540C was strongly associated with tooth shoveling (Figure 4): Spearman's rho between the number of 1540C copies and the shoveling grade was 0.37 (p = 1.2 × 10−4) in the Tokyo samples, 0.46 (p = 2.1 × 10−6) in the Sakishima samples, and 0.42 (p = 7.7 × 10−10) overall (Table 1). The correlation coefficients (R) showed similar values. The averages of shoveling grade were 1.93 for TT, 2.52 for CT, and 3.26 for CC genotype, which suggests the additive effect of the 1540C allele. A regression analysis indicated that a 1540C allele increases 0.7 in the score of shoveling grade (Table 2). The coefficient of determination was 0.189, which means that 18.9% of the variance was explainable by the EDAR variants. The neighboring synonymous SNP, C1487T, was less correlated with the shoveling in the combined samples (rho = 0.36, p = 1.8 × 10−7). The EDAR T1540C genotype was also correlated with the double-shoveling grade (rho = 0.39, p = 1.2 × 10−8), mesiodistal diameter of U1 (rho = 0.20, p = 5.4 × 10−3), PC1 (rho = 0.20, p = 4.9 × 10−3), and PC2 (rho = −0.21, p = 2.6 × 10−3) (Table 1 and Figure 5). Multiple-regression analyses considering the effects of sex and region as explanatory variables confirmed the significant association of EDAR T1540C with dental morphology (Table 2).
Figure 4.
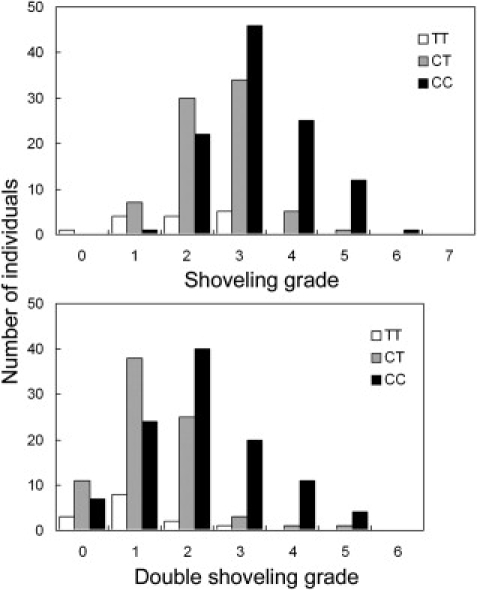
Distribution of Shoveling and Double-Shoveling Grades in Each Genotype
The white bar indicates TT, the gray bar indicates CT, and the black bar indicates CC.
Table 2.
Multiple-Regression Analyses for Dental Morphology
|
Partial Regression Coefficient |
p Value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sexa | Region | EDAR T1540C | Constant | Sexa | Region | EDAR T1540C | R2 | |
| SHb | - | - | 0.702 | 3.251 | - | - | 1.5E-10 | 0.189 |
| SH | 0.191 | −0.058 | 0.677 | 3.139 | 1.8E-01 | 6.6E-01 | 8.8E-10 | 0.199 |
| DSH | 0.415 | 0.190 | 0.626 | 1.719 | 1.2E-02 | 2.2E-01 | 6.5E-07 | 0.161 |
| UI1MD | −0.199 | 0.152 | 0.203 | 8.749 | 9.3E-03 | 3.3E-02 | 4.3E-04 | 0.110 |
| PC1 | - | 0.970 | 1.085 | 0.102 | - | 3.8E-02 | 4.1E-03 | 0.057 |
| PC2 | - | −0.052 | −0.530 | −0.255 | - | 8.1E-01 | 1.8E-03 | 0.048 |
| PC3 | - | 0.017 | 0.148 | 0.025 | - | 9.3E-01 | 3.1E-01 | 0.005 |
| PC4 | - | 0.094 | 0.195 | −0.047 | - | 5.2E-01 | 9.9E-02 | 0.015 |
The following abbreviations are used: SH, shoveling grade; DSH, double-shoveling grade; UI1, upper first incisor; MD, mesiodistal diameter; and R2, coefficient of determination. p values less than 0.01 are shown in bold.
Sex was not included in the regression analyses for PCs because PCA was performed after standardization in each sex.
Only EDAR T1540C was used as explanatory variable.
Figure 5.
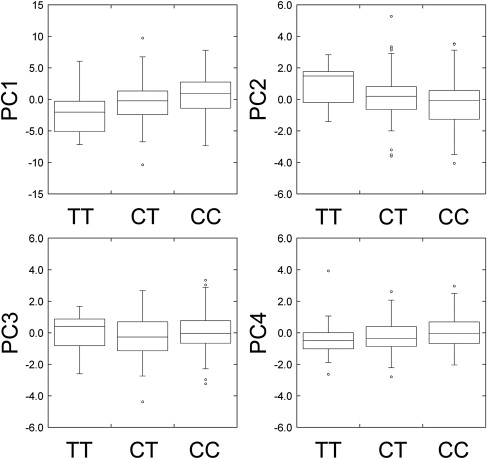
EDAR T1540C Genotypes and Principal Components
The box plots depict minimum, lower quartile (Q1), median (Q2), upper quartile (Q3), maximum, and, if any, outliers for each genotype.
A recent study of genome diversity has suggested that Japanese people fall into two main genetic clusters, named “Hondo” (denoting the main islands including Honshu, Hokkaido, Kyushu, and Shikoku) and “Ryukyu” (which includes the Sakishima Islands), and the allele frequencies for EDAR T1540C differ greatly between the clusters.31 To evaluate the effect of population stratification, we genotyped five other SNPs (rs555766, rs10495392, rs17075469, rs17265387, and rs2071652) that have shown the highest differentiation between the two clusters31 by using Taqman assays. Unlike EDAR T1540C, some of these SNPs showed significantly different frequencies between our sample sets from Tokyo and the Sakishima Islands (Table S4). As for the association between the SNPs on the various chromosomal regions, a marginally significant correlation (p < 0.05) was observed only between EDAR T1540C and rs17265387 (R = 0.143); these SNPs are on chromosome 2 but span more than 90 Mb distance. However, no SNP was significantly associated with dental morphology except for EDAR T1540C, which indicates that population stratification, if any, had little effect on our association study using the Japanese populations.
The present study shows that shoveling and double-shoveling grades are positively correlated with the mesiodistal diameter of UI1. These correlations are also supported by dental characters of Homo erectus and Neanderthals, which have wide, shovel-shaped incisors.9,32 These observations might suggest that overgrowth of upper incisors is involved in the shovel shape. However, in contrast to the fact that males generally have larger tooth size than females, we observed that the double-shoveling grade in females is significantly greater than that in males, and the shoveling grade shows the same tendency. This sexual difference is consistent with some previous studies.33,34 Although the reason for this is unclear, it could be related to the mechanisms of morphogenesis; genes on sex chromosomes, the levels of sex hormones, the time and duration of development, and/or the capacity of the maxillary dental arch might partly participate in the formation of tooth shoveling. Further studies should be undertaken to investigate this point. In the comparison between the regions, we found that the Sakishima females have a larger tooth size than the Tokyo females. The regional difference was particularly strong in the mesiodistal diameter of canines, which generally display a marked sexual dimorphism as shown in the present and previous studies.35 These results imply that dental morphology in Sakishima females might be slightly virilized.
As a result of data reduction with PCA, we found that individual variations can be efficiently summarized by four PCs. PC1 represents the overall tooth size, occupying almost half of the proportion of variance in the PCA (45.9%). More intriguing is the occurrence of the axes for the balances of buccolingual versus mesiodistal diameters (PC2), anterior versus posterior sizes (PC3), and molar versus premolar sizes (PC4), which might imply the presence of developmental machineries involved in these axes. These results are concordant with those of Harris and Bailit, in which 2,650 individuals from the Solomon Islands were studied.36 Therefore, we suggest that these components, which possibly reflect biologically meaningful entities, are robust and generalize to human populations worldwide.
This study clearly demonstrated that, for the Asian-specific EDAR 1540C allele, the number of copies is strongly correlated with the tooth-shoveling grade (rho = 0.42, p = 7.7 × 10−10). The EDAR 1540C variant explains 18.9% of the total variance in the shoveling grade, which corresponds to about one-fourth of the heritability (approximately 0.75) reported previously.5,11,13 Although a neighboring synonymous SNP, C1487T, is also significantly associated with the tooth-shoveling grade, the smaller correlation coefficient (rho = 0.36, p = 1.8 × 10−7) suggests that the apparent association is due to the linkage disequilibrium with the causative variant. The significant associations of EDAR T1540C with PC1 and PC2, as well as with the size of UI1, indicate that the SNP affects the overall tooth size and the ratio of mesiodistal diameter to buccolingual diameter. Previous studies have investigated the frequency of EDAR 1540C in global populations, which implies its correlation with the prevalence of the tooth shoveling.15 It is inferred from these observations that this nonsynonymous SNP in EDAR explains not only variation among individuals but also differences among populations in tooth shoveling. The distribution of Sinodonty can be partly attributed to the distribution of the EDAR 1540C allele.
A genome-wide study of 140,387 SNPs demonstrated that 7,003 Japanese individuals could be clearly divided into “Hondo” and “Ryukyu” clusters without prior information about which population they belong to, and the same study pointed out that the frequency of EDAR 1540C is relatively differentiated between the two clusters (Hondo: 0.778; Ryukyu: 0.602).31 Therefore, like tooth shoveling, EDAR T1540C can actually be used as an informative ethnic marker not only within Asia but also within Japan. In this study, the frequency of 1540C in the Sakishima Islands (0.715), which are located within the Ryukyu Islands, is intermediate between those in the Hondo and the Ryukyu clusters and showed no significant difference from that in the Tokyo samples (0.755), which is consistent with the lack of difference in tooth-shoveling grade between the two sample sets. However, an analysis of short-tandem-repeat polymorphisms on autosomes has indicated that the Sakishima Islanders genetically form the Ryukyu group together with the Okinawa Islanders.37 This is an example showing that a single locus, even if it is an informative ethnic marker, provides only limited information. Local genetic drift can yield variable allele frequencies between local populations within Ryukyu Islands. In contrast, the ensemble of dozens of informative markers can discriminate Ryukyu populations from Hondo populations, and, furthermore, the ensemble of thousands of markers can discriminate the Ryukyu individuals from Hondo individuals.
In previous studies, EDAR T1540C has been shown to be associated with hair thickness in Asian populations.25,26 Although it is generally known that both the thick hair and shovel-shaped incisors are phenotypes specific to Asian populations with a south-to-north increasing cline,4 it has never been reported that the two traits are correlated at the individual level. Our study does not directly demonstrate a correlation between the two traits but indicates it through finding their common genetic factor. Recent studies based on evolutionary genetics have supported the idea that the increase of the EDAR 1540C frequency in Asian populations is due to positive selection rather than genetic drift.14,15,26,38 Because the 1540C variant is absent in the European and African populations, it is thought that the variant occurred in an ancestral Asian population after the divergence from ancestral European populations and increased by positive selection on the variant itself rather than by hitchhiking on another neighboring locus under selection. However, there are enigmatic questions still remaining: what is the true selective pressure? What is the true subject of positive selection? The larger tooth size and the shovel shape of incisors result in greater structural strength, which might have been adaptive in a certain culture in which people use their teeth as tools. Alternatively, positive selection might have acted on hair structure, and tooth shoveling might be a by-product. Potentially, because the EDA-EDAR pathway affects several organs of ectodermal origin, other unexamined traits, such as in sweat and mammary glands,22 could be the true subject of selection.
The EDAR V370A polymorphic residue is located within the EDAR death domain, which is a potential region of interaction with other proteins, such as EDAR-associated death domain (EDARADD [MIM 606603]).15 In addition, the death domain has been suggested to affect EDAR's signaling activity. However, results of previous reporter-gene assays are controversial; Fujimoto et al.26 observed that 370A reduced EDAR signal output, whereas more recent studies yielded the opposite result;39,40 the authors of these more recent publications argue that Fujimoto et al. might have observed reduced EDAR signal because their assay conditions (a high dose of plasmid DNA and a long [48 hr] posttransfection incubation period) might have lead to cell death. Indeed, it has been reported that transgenic mice with elevated Eda or Edar activity have a thicker hair than wild-type mice, thus mimicking the East Asian hair phenotype.22,40 As for dental morphology, the overexpression of Edar results in molars with extra cusps and supernumerary teeth,20 and the overexpression of Eda results in molars of abnormal shape and incisors lacking enamel formation;22 these phenotypes do not mimic the shovel-shaped incisors observed in humans. Because animal models do not always replicate human phenotypes, there might be difficulty in clarifying the mechanisms for how the EDAR polymorphism is involved in the formation of shovel-shaped incisors.
We revealed a genetic determinant of tooth shoveling, which has received great attention from dental anthropologists. However, because the EDAR polymorphism alone cannot explain the heritability of tooth shoveling suggested in previous studies, we still need to seek other genetic factors. In general, many dental traits still need to be examined to identify the associated genes, for example, Carabelli's tubercle, the numbers of cusps and roots, and the patterns of tooth size shown in the PCA. Further studies using powerful DNA technology will provide knowledge on the associations between genotypes and phenotypes in dental traits, which contribute to the basic biological and medical sciences.
Acknowledgments
We are deeply grateful to the people participating in this study. We also thank Y. Tomoyasu for her assistance in collecting samples at the Showa University Dental Hospital. This study is partly supported by the Rising Star Program for Subtropical Island Sciences of the University of the Ryukyus and by a Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (JSPS).
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
GenBank, http://ncbi.nlm.nih.gov/Genbank/
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
- 1.Hanihara T. Morphological variation of major human populations based on nonmetric dental traits. Am. J. Phys. Anthropol. 2008;136:169–182. doi: 10.1002/ajpa.20792. [DOI] [PubMed] [Google Scholar]
- 2.Hanihara K. Racial characteristics in the dentition. J. Dent. Res. 1967;46:923–926. doi: 10.1177/00220345670460055101. [DOI] [PubMed] [Google Scholar]
- 3.Turner C.G., 2nd Major features of Sundadonty and Sinodonty, including suggestions about East Asian microevolution, population history, and late Pleistocene relationships with Australian aboriginals. Am. J. Phys. Anthropol. 1990;82:295–317. doi: 10.1002/ajpa.1330820308. [DOI] [PubMed] [Google Scholar]
- 4.Mizoguchi Y. University of Tokyo Press; Tokyo: 1985. Shovelling: A statistical analysis of its morphology. Bulletin 26, The Univeristy Museum. [Google Scholar]
- 5.Scott G.R., Turner C.G., 2nd . Cambridge University Press; Cambridge, UK: 1997. The Anthropology of Modern Human Teeth: Dental Morphology and Its Variation in Recent Human Populations. [Google Scholar]
- 6.Brace C.L., Nagai M. Japanese tooth size: Past and present. Am. J. Phys. Anthropol. 1982;59:399–411. doi: 10.1002/ajpa.1330590410. [DOI] [PubMed] [Google Scholar]
- 7.Turner C.G., 2nd Dental anthropological indications of agriculture among the Jomon people of central Japan. X. Peopling of the Pacific. Am. J. Phys. Anthropol. 1979;51:619–636. [Google Scholar]
- 8.Hanihara T., Ishida H. Metric dental variation of major human populations. Am. J. Phys. Anthropol. 2005;128:287–298. doi: 10.1002/ajpa.20080. [DOI] [PubMed] [Google Scholar]
- 9.Weidenreich, F. (1937). The dentition of Sinanthropus pekinensis: A comparative odontography of the hominids. Palaeontolagia Sinica, New Series D, Whole Series No. 101, No. 1.
- 10.Wolpoff M.H. Multiregional evolution: The fossil alternative to Eden. In: Stringer C.B., editor. The Human Revolution. Edinburgh University Press; Edinburgh, UK: 1989. pp. 62–108. [Google Scholar]
- 11.Hanihara K., Masuda T., Tanaka T. Family studies of the shovel trait in the maxillary central incisor. Journal of the Anthropological Society of Nippon. 1974;83:107–112. [Google Scholar]
- 12.Mizoguchi Y. Genetic variability in tooth crown characters: Analysis by the tetrachoric correlation method. Bulletin of the National Science Museum. Series D, Anthropology. 1977;3:37–62. [Google Scholar]
- 13.Blanco R., Chakraborty R. The genetics of shovel shape in maxillary central incisors in man. Am. J. Phys. Anthropol. 1976;44:233–236. doi: 10.1002/ajpa.1330440204. [DOI] [PubMed] [Google Scholar]
- 14.Kimura R., Fujimoto A., Tokunaga K., Ohashi J. A practical genome scan for population-specific strong selective sweeps that have reached fixation. PLoS ONE. 2007;2:e286. doi: 10.1371/journal.pone.0000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabeti P.C., Varilly P., Fry B., Lohmueller J., Hostetter E., Cotsapas C., Xie X., Byrne E.H., McCarroll S.A., Gaudet R. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449:913–918. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The International HapMap Consortium A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monreal A.W., Ferguson B.M., Headon D.J., Street S.L., Overbeek P.A., Zonana J. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat. Genet. 1999;22:366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- 18.Tucker A.S., Headon D.J., Schneider P., Ferguson B.M., Overbeek P., Tschopp J., Sharpe P.T. Edar/Eda interactions regulate enamel knot formation in tooth morphogenesis. Development. 2000;127:4691–4700. doi: 10.1242/dev.127.21.4691. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Ullrich R., Aebischer T., Hulsken J., Birchmeier W., Klemm U., Scheidereit C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128:3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- 20.Tucker A.S., Headon D.J., Courtney J.M., Overbeek P., Sharpe P.T. The activation level of the TNF family receptor, Edar, determines cusp number and tooth number during tooth development. Dev. Biol. 2004;268:185–194. doi: 10.1016/j.ydbio.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Pispa J., Mustonen T., Mikkola M.L., Kangas A.T., Koppinen P., Lukinmaa P.L., Jernvall J., Thesleff I. Tooth patterning and enamel formation can be manipulated by misexpression of TNF receptor Edar. Dev. Dyn. 2004;231:432–440. doi: 10.1002/dvdy.20138. [DOI] [PubMed] [Google Scholar]
- 22.Mustonen T., Pispa J., Mikkola M.L., Pummila M., Kangas A.T., Pakkasjarvi L., Jaatinen R., Thesleff I. Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev. Biol. 2003;259:123–136. doi: 10.1016/s0012-1606(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 23.Fraser G.J., Bloomquist R.F., Streelman J.T. A periodic pattern generator for dental diversity. BMC Biol. 2008;6:32. doi: 10.1186/1741-7007-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris M.P., Rohner N., Schwarz H., Perathoner S., Konstantinidis P., Nusslein-Volhard C. Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates. PLoS Genet. 2008;4:e1000206. doi: 10.1371/journal.pgen.1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto A., Ohashi J., Nishida N., Miyagawa T., Morishita Y., Tsunoda T., Kimura R., Tokunaga K. A replication study confirmed the EDAR gene to be a major contributor to population differentiation regarding head hair thickness in Asia. Hum. Genet. 2008;124:179–185. doi: 10.1007/s00439-008-0537-1. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto A., Kimura R., Ohashi J., Omi K., Yuliwulandari R., Batubara L., Mustofa M.S., Samakkarn U., Settheetham-Ishida W., Ishida T. A scan for genetic determinants of human hair morphology: EDAR is associated with Asian hair thickness. Hum. Mol. Genet. 2008;17:835–843. doi: 10.1093/hmg/ddm355. [DOI] [PubMed] [Google Scholar]
- 27.Toma T., Hanihara T., Sunakawa H., Haneji K., Ishida H. Metric dental diversity of Ryukyu Islanders: A comparative study among Ryukyu and other Asian populations. Anthropol. Sci. 2007;119:119–131. [Google Scholar]
- 28.Haneji K., Hanihara T., Sunakawa H., Toma T., Ishida H. Nonmetric dental variation of Sakishima Islanders, Okinawa, Japan: A comparative study among Sakishima and neighboring populations. Anthropol. Sci. 2007;115:119–131. [Google Scholar]
- 29.Turner C.G., 2nd, Nichol C.R., Scott G.R. Scoring procedures for key morphological traits of the parmanent dentition. ASU Dental Anthropology System. In: Larsen C.S., editor. Advances in Dental Anthropology. Wiley-Liss; New York: 1991. pp. 13–32. [Google Scholar]
- 30.Higa T., Hanihara T., Sunakawa H., Ishida H. Dental variation of Ryukyu islanders: A comparative study among Ryukyu, Ainu, and other Asian populations. Am. J. Hum. Biol. 2003;15:127–143. doi: 10.1002/ajhb.10138. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi-Kabata Y., Nakazono K., Takahashi A., Saito S., Hosono N., Kubo M., Nakamura Y., Kamatani N. Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: Effects on population-based association studies. Am. J. Hum. Genet. 2008;83:445–456. doi: 10.1016/j.ajhg.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey S.E. A closer look at Neanderthal postcanine dental morphology: The mandibular dentition. Anat. Rec. 2002;269:148–156. doi: 10.1002/ar.10116. [DOI] [PubMed] [Google Scholar]
- 33.Harris E.F. Sex differences in lingual marginal ridging on the human maxillary central incisor. Am. J. Phys. Anthropol. 1980;52:541–548. doi: 10.1002/ajpa.1330520411. [DOI] [PubMed] [Google Scholar]
- 34.Rothhammer F., Lasserre E., Blanco R., Covarrubias E., Dixon M. Microevolution in human Chilean populations. IV. Shovel shape, mesial-palatal version and other dental traits in Pewenche Indians. Z. Morphol. Anthropol. 1968;60:162–169. [PubMed] [Google Scholar]
- 35.Garn S.M., Cole P.E., Wainwright R.L., Guire K.E. Sex discriminatory effectiveness using combinations of permanent teeth. J. Dent. Res. 1977;56:697. doi: 10.1177/00220345770560062601. [DOI] [PubMed] [Google Scholar]
- 36.Harris E.F., Bailit H.L. A principal components analysis of human odontometrics. Am. J. Phys. Anthropol. 1988;75:87–99. doi: 10.1002/ajpa.1330750110. [DOI] [PubMed] [Google Scholar]
- 37.Matsukusa H., Oota H., Haneji K., Toma T., Kawamura S., Ishida H. A genetic analysis on the Sakishima Islanders reveals no relationship with Taiwan aborigines but Ainu and main-island Japanese. Am. J. Phys. Anthropol. 2010 doi: 10.1002/ajpa.21212. in press. [DOI] [PubMed] [Google Scholar]
- 38.Carlson C.S., Thomas D.J., Eberle M.A., Swanson J.E., Livingston R.J., Rieder M.J., Nickerson D.A. Genomic regions exhibiting positive selection identified from dense genotype data. Genome Res. 2005;15:1553–1565. doi: 10.1101/gr.4326505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bryk J., Hardouin E., Pugach I., Hughes D., Strotmann R., Stoneking M., Myles S. Positive selection in East Asians for an EDAR allele that enhances NF-kappaB activation. PLoS ONE. 2008;3:e2209. doi: 10.1371/journal.pone.0002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mou C., Thomason H.A., Willan P.M., Clowes C., Harris W.E., Drew C.F., Dixon J., Dixon M.J., Headon D.J. Enhanced ectodysplasin-A receptor (EDAR) signaling alters multiple fiber characteristics to produce the East Asian hair form. Hum. Mutat. 2008;29:1405–1411. doi: 10.1002/humu.20795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


