Abstract
The causal role of wood-dust exposure in sinonasal cancer (SNC) has been established in epidemiological studies, but the mechanisms of SNC carcinogenesis are still largely unknown. Increased amounts of COX-2 are found in both premalignant and malignant tissues, and experimental evidence link COX-2 to development of cancer. Many signals that activate COX-2 also induce tumour suppressor p53, a transcription factor central in cellular stress response. We investigated COX-2 and p53 expressions by immunohistochemistry in 50 SNCs (23 adenocarcinomas, and 27 squamous cell carcinomas (SCC); 48 analysed for COX-2; 41 for p53). Occupational histories and smoking habits were available for majority of the cases. Most of the adenocarcinoma cases with exposure history data had been exposed to wood-dust at work in the past (88 %, 14/16). For smokers, 63 % (12/19) presented with SSC, whereas 64 % (7/11) of non-smokers displayed adenocarcinoma. COX-2 was expressed at high levels in adenocarcinoma as compared to SSC (p < 0,001). COX-2 expression showed significant association with occupational exposure to wood dust (p = 0.024), and with non-smoking status (p = 0.001). No statistically significant associations between the exposures and p53 accumulation were found; however, the p53 accumulation pattern (p = 0.062 for wood dust exposure) resembled that of COX-2 expression. In summary, our findings show increased COX-2 expression in SNC adenocarcinoma with wood dust exposure, suggesting a role for inflammatory components in the carcinogenesis process. In contrast, SCCs predominated among smokers and expressed COX-2 rarely; this may suggest at least partially different molecular mechanisms.
Keywords: Adenocarcinoma; etiology; metabolism; Carcinoma, Squamous Cell; metabolism; Cyclooxygenase 2; metabolism; Dust; Gene Expression Regulation, Enzymologic; Gene Expression Regulation, Neoplastic; Humans; Immunohistochemistry; Nose Neoplasms; etiology; metabolism; Occupational Exposure; adverse effects; Paranasal Sinus Neoplasms; etiology; metabolism; Tumor Markers, Biological; metabolism; Tumor Suppressor Protein p53; metabolism; Wood
Keywords: sinonasal cancer, COX-2 expression, p53 expression, wood dust exposure, smoking
Introduction
Cancer of the nose and paranasal sinuses is a rare form of cancer, with an incidence of 0.5 to 1.5 new cases per year per 100 000 in men and 0.1 to 0.6 per 100 000 in women. However, the incidence of this cancer varies markedly from one country to the next, and, even between different parts of the same country. This variation has not been attributed to individual susceptibility, such as genetic differences, but rather to differences in exposure 1, 2. Numerous epidemiological studies have established a causal role of exposure to wood dust in the development of sinonasal cancer, with extremely high relative risks, up to 10–30-fold, indicated for the sinonasal adenocarcinoma cell type 2, 3.
Multiple mechanisms of carcinogenesis have been proposed to be involved in the development of SNC, but with very little experimental or human data. Genotoxicity has been attributed to the chemical components of wood as well as to the physico-mechanical properties of wood-dust particles, but the data are in general sparse 2. Also, inflammation has been suggested to play a significant role in SNC carcinogenesis. In addition to experimental studies where wood dust has been shown to elicit an inflammatory response 4–6, there are epidemiological studies revealing that occupational exposure to dusts from various wood species is associated with asthma 7. There are also consistent reports of impaired mucociliary clearance and mucosal alterations during chronic exposure 2. Mucosal alterations include dysplasia and metaplasia of the columnar epithelium, and, to a lesser extent, changes in the squamous epithelium 2, 7.
There is considerable evidence from genetic, pharmacological and clinical studies to link COX-2 to the development of cancer. Increased amounts of COX-2, in comparison to normal tissues, are found commonly in both premalignant and malignant tissues, and inhibitors of COX, such as aspirin or other non-steroidal anti-inflammatory drugs, have been shown to reduce the incidence of different malignancies 8–11. Expression of COX-2 is induced by many physiological and stress signals including growth factors, cytokines and other mediators of inflammation, tumour promoters, oxidizing agents and DNA damaging agents 12. Many of the signals that activate COX-2 also induce tumour suppressor p53. The p53 is a transcription factor that induces antiproliferative responses such as cell cycle arrest, DNA repair, or apoptosis in response to DNA damage 12. The p53 pathway is disturbed in practically all main types of human cancer 13
The present study was designed to investigate the expression of COX-2 in a series of sinonasal carcinomas. We sought to determine whether the pattern of COX-2 expression showed an association with tumour histology, wood-dust exposure, or smoking. In addition, we investigated p53 expression in the tumour tissue, to analyse possible parallel patterns of exposure between COX-2 and p53. The overall goal of the study was to examine the involvement of inflammation in the tumorigenesis process of SNC. To our knowledge, COX-2 expression has not been studied before in sinonasal carcinoma.
Material and Methods
Patients and samples
The sample set consisted of 50 sinonasal cancers (ICD-10: C30.0, C31; ICD-9: 160 except 160.1) cases from Finland and France. COX-2 staining was performed for 48 samples and p53 for 41 samples. Archival tumour specimens were collected in collaboration with the Finnish cancer registry and three French cancer registries (in the areas of Isère, Somme and Doubs) as part of a larger multi-centre study investigating the biological effects of wood-dust exposure. All SNC cases included were of Caucasian origin, with 19 cases collected in the Finnish study centre (Finnish Institute of Occupational Health, Helsinki) and 31 in the French counterpart (Inserm, St. Maurice). Of the tumours, 13 were situated in ethmoid sinus, 12 in sinus maxillaris sinus, 22 in nasal cavity, and 3 in an unspecified sinus.
A panel of three pathologists (MD, TS, HW) reviewed the histopathological diagnoses of the tumour sections, in order to achieve consistent diagnostic criteria throughout the series. The tumour histologies included in the study were adenocarcinoma (n = 23) and squamous cell carcinoma (SCC, n= 27) cell types. Adenocarcinomas were further subclassified for intestinal type adenocarcinoma (ITAC) or non-ITAC. ITAC was composed of intestinal-type epithelium with or without mucin production in contrast to the restrictive WHO definition where only adenocarcinomas with both intestinal-type epithelium and mucin production are considered to be ITAC14. Of the total of 23 adenocarcinomas studied, 12 were ITAC according to the WHO classification, whereas 22 represented the intestinal subtype according to our classification. Altogether 12 adenocarcinomas were of ethmoidal origin.
Patients or next-of-kins for deceased patients were interviewed to collect information on smoking and occupational exposure to wood dust; for the Finnish cases, this information was supplemented with data from the Finnish Centre for Pensions. Wood dust exposure at work was assessed by an industrial hygienist (PH), with consultations with two other industrial hygienists experienced in wood processing industry. There were 17 wood dust -exposed cases and 15 non-exposed; with information unavailable for 18 cases. In all, 19 cases were smokers and 11 non-smokers, with information missing in 20 cases. The study was approved by the appropriate national Ethical Review Boards in Finland and France.
Immunohistochemistry analysis
COX-2 expression
Sections (4 μm) cut from paraffined tissue blocks on coated slides were incubated overnight at 37 °C, deparaffinized and then microwaved for 4 × 5 min in 0.01 Mmm Na-citrate buffer (pH 6.0). To block endogenous peroxidase activity, the slides were immersed in 1.6 % hydrogen peroxide in methanol for 30 min and then in blocking solution for unspecific binding sites (0.5% BSA and 1.5 % normal serum in PBS). Immunostaining was performed by a similar protocol as described earlier 15, with monoclonal IgG against human COX-2 protein peptide (Cayman Chemical Co.) at a dilution of 1:100 overnight at room temperature. After this, the slides were treated with biotinylated secondary antibody (anti-mouse IgG) at a dilution of 1:250 (Vectastain Elite ABC kit PK-6102, Vector Laboratories) in solution of 0.5 % BSA in PBS for 30 min. Antibody binding sites were visualized with avidin-biotin peroxidase complex solution (Vectastain Elite kit PK-6102, Vector Laboratories), after treatment for 30 min, and AEC (3-Amino-9-ethylcarbazole) liquid for 15 min. Counterstaining was done by Mayer hematoxylin. The COX-2 index was calculated by multiplying the percentage of COX-2 positive cells by the intensity (1–5) of the COX-2 staining. Cases with intensity 1 or COX-2 index less than 40 were considered as negative (−), indexes 41–200 were scored as weak (+), 201–300 as moderate (++) and over 300 as strong (+++).
p53 accumulation
Sections (4 μm) cut from paraffined tissue blocks on coated slides were incubated overnight at 37 °C, deparaffinized and then microwaved 4 × 5 min in 0.01 Mmm Na-citrate buffer (pH 6.0). Immunohistochemistry (IHC) staining was performed using the Ventana Benchmark automated immunostainer (Tucson Medical Systems) following the protocols provided by the manufacturer. The antibody used was DO-7 for wild type p53. The p53 nuclear accumulation was scored as follows: if nuclear staining was seen in only 0–1 % of the cells, the case was considered as negative (−), in 2–33 % as weak (+), in 33–79 % as moderate (++) and over 80 % as strong (+++).
COX-2 expression by real time quantitative PCR
To further confirm the COX-2 expression detected by IHC, we performed a real-time quantitative PCR analysis. RNA was successfully extracted from 10 adenocarcinomas by using High Pure RNA Paraffin kit (Roche) according to manufacturer’s instructions. The RNA extraction was successful for a subset of adenocarcinomas; the rest of the tissue samples were either too small or possibly too old to yield RNA of acceptable quality. The cDNA synthesis and real-time quantitative PCR (Taqman assay) with an AbiPrism 7700 Sequence Detector System (Applied Biosystems) were performed as described earlier 16. PCR primers and probes designed by Applied Biosystems and purchased from the company were used. The results were expressed as relative units (RU), which were calculated by the comparative CT method 16.
Statistical analysis
Statistical analyses were performed using the Stata software (Stata statistical software: Release 9. College Station, TX: Statacorp LP 2005), A score ++ or +++ was considered positive. Fisher’s exact test (two-sided) was used to compare proportions of positive cases.
Results
COX-2
Altogether 48 SNC tumours were analysed for COX-2 expression by immunohistochemistry. A summary of IHC results on COX-2 and p53 is shown in Table 1, and representative examples of COX-2 expression in sinonasal cancer are illustrated in Figure 1. We found that expression of COX-2 was statistically significantly associated with the adenocarcinoma histology (p < 0,001, Table 1; Figure 2). In SCCs, COX-2 was poorly or not at all expressed, whereas in adenocarcinomas, a strong COX-2 expression was seen. All but one (92 %; 12/13) of the adenocarcinoma tumours exhibiting moderate or strong COX-2 expression were ITAC. Interestingly, COX-2 expression was stronger in non-smokers (p = 0.001) and in the wood dust -exposed subjects (p= 0.024), as shown in Table 1 and Figure 3. No association between COX-2 expression and tumour site (Table 1) or tumour grade (data not shown) was observed.
Table 1.
COX-2 and p53 expressions (immunohistochemistry) in sinonasal cancer.
| Tumour/patient characteristic | COX-2 −/+* | COX-2 ++/+++* | P (Fisher exact test) | p53 −/+* | p53 ++/+++* | P (Fisher exact test) |
|---|---|---|---|---|---|---|
| Histology | ||||||
| Adenocarcinoma | 9 | 13 | <0.001 | 9 | 10 | |
| Squamous cell carcinoma | 24 | 2 | 11 | 11 | 1.00 | |
| Tumour site | ||||||
| Ethmoid | 6 | 7 | 4 | 9 | ||
| Nasal Cavity | 16 | 6 | 9 | 8 | ||
| Maxillary sinus | 11 | 2 | 0.13 | 7 | 4 | 0.30 |
| Smoking | ||||||
| Ever | 16 | 2 | 8 | 7 | 1.00 | |
| Never | 3 | 8 | 0.001 | 5 | 5 | |
| Wood dust exposure | ||||||
| Yes | 7 | 10 | 5 | 9 | ||
| No | 12 | 2 | 0.02 | 9 | 5 | 0.06 |
+ = weak expression; ++ = moderate expression; +++ = strong expression; − = no expression.
Figure 1. Representative examples of COX-2 expression in sinonasal cancer.
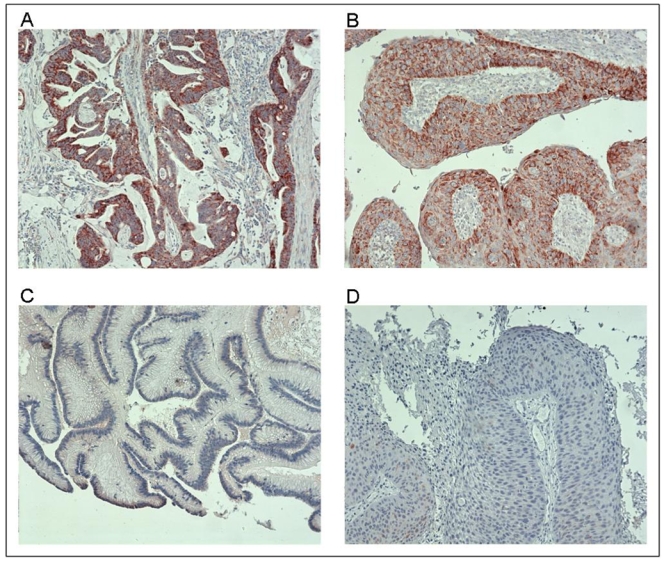
A) strong expression of COX-2 (+++) in ethmoidal adenocarcinoma; B) moderate expression of COX-2 (++) in SCC from nasal septum; C) no expression of COX-2 (−) in ethmoidal adenocarcinoma; D) no expression in SCC from nasal cavity. The original magnification was 100× in each example (A–D).
Figure 2. Distribution of COX-2 expression according to tumour histology in sinonasal cancer.
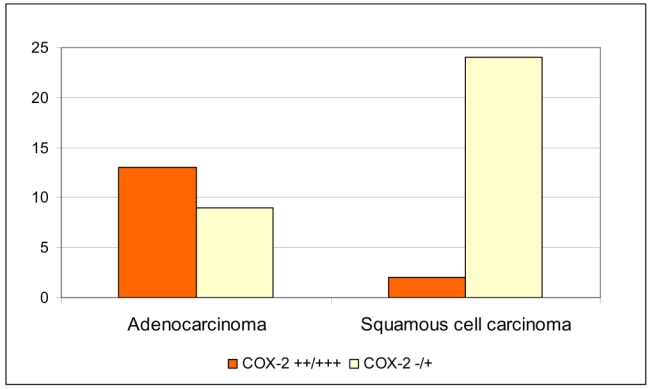
(−/+): weak or no expression of COX-2; (++/+++): moderate or strong expression of COX-2.
Figure 3. Distribution of COX-2 expression in sinonasal carcinomas by exposure and tumour histology.
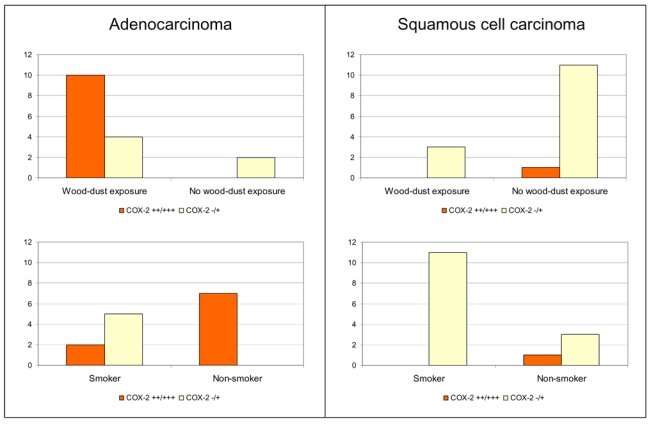
(−/+): weak or no expression of COX-2; (++/+++): moderate or strong expression of COX-2.
COX-2 expression could also be detected at mRNA level from the tumour tissue by real time quantitative PCR analysis. This analysis was performed only on a subset of adenocarcinomas due to limitations with tissue material available. The levels of COX-2 mRNA expression determined by the real time quantitative PCR assay showed variation between the tumours (range 6 – 384 RU; Figure 4) and were in parallel with the expression seen in IHC in most cases (Pearson correlation coefficient between IHC index and RU was 0.63).
Figure 4. Comparison between COX-2 mRNA expression (real time quantitative PCR assay) and COX-2 immunohistochemisty (IHC) in sinonasal adenocarcinoma.
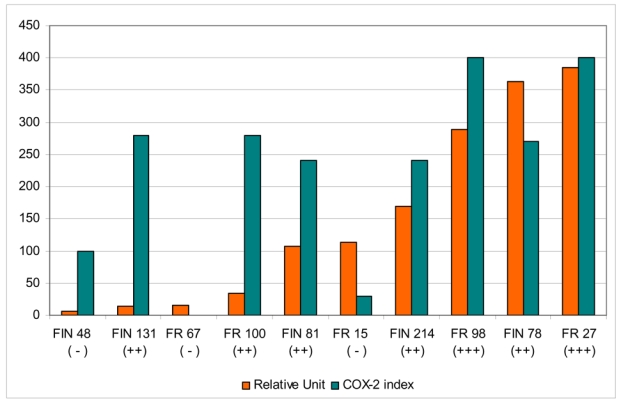
Real time quantitative PCR results are expressed as relative units, and IHC results as COX-2 index (percentage of COX-2 positive cells x intensity of COX-2 staining). The +/− sign in parentheses under the tumour codes mark the IHC classification as in Figures 3 and 4.
p53
Overall, about half of the 41 cases that underwent p53 IHC analysis expressed p53 (Table 1). No statically significant associations were found; however, some tendencies could be seen. Collectively, the SNC cases with wood-dust exposure in the past more often exhibited strong staining for p53 as compared with the non-exposed (p = 0.062; Table 1), resembling the staining pattern observed for COX-2 expression. In the adenocarcinomas, all the more strongly positive p53 accumulations (levels ++/+++) were found in non-smokers (p = 0.061) and wood dust -exposed cases (p=0.15). In SCC, on the contrary, smokers tended to show more p53 accumulation (from cases with p53 accumulation levels of ++/+++ and data available for smoking history, 7/7 were smokers). Unlike COX-2, p53 accumulation was not associated with tumour histology (Table 1). The association between p53 and COX-2 expressions was close to statistical significance in adenocarcinomas (p = 0.054) but not in SCCs.
Associations with exposure and tumour histology
From the 17 SNC cases with work-related wood dust exposure in the past, 82 % (14/17) presented with adenocarcinoma (all ITAC) and 18 % (3/17) with SCC. The association between the histological tumour type and wood dust exposure was statistically significant (p <0.001). There was a non-significant association between smoking and SCC; 63 % (12/19) of smokers presented with squamous cell carcinoma and 64 % (7/11) of the non-smokers displayed adenocarcinoma tumours. Smoking was not associated with wood-dust exposure. The cancer site and the histological type were also significantly associated (p<0.001; data not shown); almost all ethmoidal cancers were adenocarcinomas, whereas almost all cancers of maxillary sinus were SCCs.
Discussion
In the present study, we observed a significantly elevated COX-2 expression in human sinonasal adenocarcinoma (mostly ITAC), as compared to squamous cell carcinoma. Our IHC findings on COX-2 expression were supported by further analysis of COX-2 mRNA levels in a subset of adenocarcinoma tumours. These results are in accordance with increased COX-2 expression reported for other human cancers, such as lung cancer 11, colon cancer 17, pancreatic cancer 18 and stomach cancer 19. Earlier, COX-2 expression has been reported to be related to smoking in oral mucosa 20, but in our study smoking did not have an effect on COX-2 expression. However, we observed that COX-2 expression in SNC was statistically significantly associated with wood dust exposure.
In the scientific literature, involvement of multifactorial biological pathways in development of wood dust-associated sinonasal cancers has been proposed 2, 7. In line with this, our recent experimental work lends support for a role of wood dust exposure in eliciting inflammatory response. We showed that dusts from various wood species were able to induce the release of proinflammatory mediators in lung macrophages and pulmonary cells in vitro and in vivo. In addition, our data suggest that generation of oxygen species (ROS) is likely to be involved in the inflammatory process 4–6. We have further preliminary data indicating that wood dusts are able to induce COX-2 expression in a dose-dependent manner in mouse pulmonary macrophages in vitro (Holmila et al., unpublished data).
Interactions between COX-2 and p53 have been shown in vitro and in vivo. It has been demonstrated that p53 can upregulate COX-212, 21, 22, or suppress the transcription of COX-29, 23. Additionally, COX-2 has been observed to exhibit strong inhibitory effects on p53 transcriptional activity 21, 22. Benoit et al. 12 found a correlation between COX-2 expression and TP53 wild-type status in oesophageal adenocarcinoma with Barrett’s oesophagus as a precursor lesion, but not in SCC, evidence that the participation of p53 in the regulation of COX-2 expression in cancer may be dependent on tumour histology. They proposed also, interestingly, that chronic inflammation could represent a physio-pathological context in which p53 and the transcription factor NF-kappaB could cooperate to activate COX-212.
In a recent review, de Moraes et al. 24 propose that the seemingly contradictory data on the cross talks between COX-2 and p535, 21–23 can be explained by two different mechanistic scenarios, not mutually exclusive though, where the variable patterns between COX-2 and p53 expressions are dependent largely on the inflammatory context. The authors further hypothesise that both the suggested mechanisms - the one involving cooperation between NF-kappaB and p53, as well as the other one in which COX-2 activation occurs independently from those two regulators, with TP53 gene mutation co-existing as an additional independent effector - may have synergistic effects in inhibiting apoptosis. They further see that such a situation may prevail in many squamous cancers as well as in cancers where TP53 mutation occurs prior to the development of a lesion as a result of intense exposure to environmental carcinogens, exemplified by lung cancer of smokers, irrespective of histology 24. Finally, it is pointed out that most likely the activation of COX-2 as in the latter mechanistic alternative may be relevant for most tumours, including those where inflammation stress occurs 24.
Our present results suggest an association between p53 accumulation and COX-2 expression in sinonasal adenocarcinomas, with no such relation seen in SCCs. It is known that a major cause of p53 accumulation is inactivating mutation in the TP53 gene; our observations thus propose that COX-2 expression may arise through mechanisms independent of p53 in at least sinonasal tumours with adenocarcinoma histology. On the basis of the mechanisms envisaged for the interplay between COX-2 and p5324, it seems likely that partially different and partially shared conditions and regulatory events of COX-2 and p53 expressions prevail in adenocarcinoma and SCC histologies of sinonasal cancer.
Immunostaining against the tumour suppressor p53 protein indicated that accumulation of p53 is common in sinonasal tumours; about half of the cases had a high level of p53 accumulation. The two histologies did not differ in this respect. In the literature, the data on sinonasal cancer are sparse but there are a few studies that have reported accumulation of p53 in sinonasal cancer 25, 26. In the study by Valente et al. 25, the number of normal epithelial cells showing p53 nuclear accumulation was significantly higher in ethmoidal mucosa of subjects with a long history of exposure to wood dust in comparison with patients not exposed to wood dust (i.e. those who had undergone an esthetic nasal surgery). Differences between wood dust-exposed and non-exposed adenocarcinoma cases were also seen in p53 expression in normal epithelium and neoplastic cells 25. We did not find statistically significant associations between p53 accumulation and the two exposures, wood dust and smoking, possibly due to the small number of cases. There was, however, a tendency (p = 0.062) for p53 accumulation to occur more frequently in tumours from the wood dust -exposed cases as compared to those from the non-exposed. In SCC, p53 accumulation was particularly seen in the group of smokers. Overall, the frequent p53 accumulation observed in this subgroup of sinonasal cancers is in accordance with preliminary results from our on-going study on TP53 mutations conducted in a larger collection of sinonasal cancers 27.
Altogether, our present molecular biology findings in sinonasal tumours are well in line with the overall data from epidemiological studies establishing a consistent link between wood-dust exposure and sinonasal cancer 2, 7. The epidemiological data indicate highly elevated risks especially for adenocarcinoma histology, and considerably smaller risks have been found for SCC 2, 7. Our study demonstrated that 88 % of the adenocarcinoma cases for which the exposure history was known, had been exposed to wood dust at work. All of the ethmoidal adenocarcinomas included in the study had occurred in wood dust -exposed cases.
Another risk factor reported for SNC is cigarette smoking, with a less pronounced, two- to threefold increased risk of nasal cancer observed among smokers and a reduction in risk among long-term quitters. The association has been suggested to be limited to squamous cell carcinoma rather than adenocarcinoma 28, 29. In our data, almost two-thirds of smokers presented with SCC, whereas a similar proportion of non-smokers displayed adenocarcinoma tumours; the associations with tumour histology, however, remained statistically non-significant. Interestingly, COX-2 expression associated with non-smoking.
In summary, our study demonstrates that COX-2 is expressed in high level in sinonasal adenocarcinomas, whereas in SCCs, the expression is much lower. Furthermore, COX-2 expression was associated with wood dust exposure, and with non-smoking status. The accumulation of p53 was found to be common in sinonasal cancer, but no statistically significant associations with p53 and histological type or exposures were found. Our current findings suggest a role for inflammatory components in carcinogenesis of sinonasal cancer. Increased knowledge on molecular cancer mechanisms associated with carcinogenic exposures is highly needed for risk assessment; such data may eventually open prospects for prevention and treatment.
Acknowledgments
We thank Ms. Tuula Suitiala, Finnish Institute of Occupational Health, for excellent technical assistance. Ms. Tuula Luikkonen, MSc, and Ms. Ritva Degerth, MSc, both from Finnish Institute of Occupational Health, are acknowledged for expert consultations in wood dust exposure assessment. In France, we thank Annie Schmaus for the coordination of sample and data collection; Joëlle Févotte for the occupational exposure assessment; the cancer registries of Doubs (A. Danzon), Isère (F. Ménégoz †), Somme (N. Raverdy); the pathologists (F. Berger, B. Bringeon, E. Brambilla, A. Ciapa, E. Faure, M.F. Gontier, B. Kantelip, C. Lelarge, F. Nagorniewicz, M.H. Panh, G. Perrot, A. Petitjean, P. Pocachard) for providing specimens.
Financial support: EU, 5th FP (WOOD-RISK, project no. QLK4-2000-00573) and the Academy of Finland (project no. 53631).
References
- 1.IARC. Cancer incidence in five continents. IARC Sci Publ. 1997;VII(143):i–xxxiv. 1–1240. [PubMed] [Google Scholar]
- 2.IARC. Wood dust and formaldehyde. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. 1995;62:35–215. [PMC free article] [PubMed] [Google Scholar]
- 3.IARC. Cancer risk from occupational exposure to wood dust. A pooled Analysis of Epidemiological Studies. IARC Technical Report. 1998:30. [Google Scholar]
- 4.Maatta J, Lehto M, Leino M, Tillander S, Haapakoski R, Majuri ML, et al. Mechanisms of particle-induced pulmonary inflammation in a mouse model: exposure to wood dust. Toxicol Sci. 2006;93(1):96–104. doi: 10.1093/toxsci/kfl026. [DOI] [PubMed] [Google Scholar]
- 5.Long H, Shi T, Borm PJ, Maatta J, Husgafvel-Pursiainen K, Savolainen K, et al. ROS-mediated TNF-alpha and MIP-2 gene expression in alveolar macrophages exposed to pine dust. Part Fibre Toxicol. 2004;1(1):3. doi: 10.1186/1743-8977-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maatta J, Luukkonen R, Husgafvel-Pursiainen K, Alenius H, Savolainen K. Comparison of hardwood and softwood dust-induced expression of cytokines and chemokines in mouse macrophage RAW 264.7 cells. Toxicology. 2006;218(1):13–21. doi: 10.1016/j.tox.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.SCOEL. Recommendations of the Scientific Committee for Occupational Exposure Limits: Risk Assessment for Wood Dust. Luxemburg: European Commission, Employment and Social Affairs DG; 2003. [Google Scholar]
- 8.Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 2004;215(1):1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24(2):96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 10.Shibata M, Kodani I, Osaki M, Araki K, Adachi H, Ryoke K, et al. Cyclo-oxygenase-1 and -2 expression in human oral mucosa, dysplasias and squamous cell carcinomas and their pathological significance. Oral Oncol. 2005;41(3):304–12. doi: 10.1016/j.oraloncology.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58(22):4997–5001. [PubMed] [Google Scholar]
- 12.Benoit V, de Moraes E, Dar NA, Taranchon E, Bours V, Hautefeuille A, et al. Transcriptional activation of cyclooxygenase-2 by tumor suppressor p53 requires nuclear factor-kappaB. Oncogene. 2006 doi: 10.1038/sj.onc.1209579. [DOI] [PubMed] [Google Scholar]
- 13.Guimaraes DP, Hainaut P. TP53: a key gene in human cancer. Biochimie. 2002;84:83–93. doi: 10.1016/s0300-9084(01)01356-6. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugaratnam K, Sobin L. WHO Histological Classification of Tumours of the Upper Respiratory Tract and Ear. 2. Springer verlag; 1991. [DOI] [PubMed] [Google Scholar]
- 15.Siironen P, Ristimaki A, Nordling S, Louhimo J, Haapiainen R, Haglund C. Expression of COX-2 is increased with age in papillary thyroid cancer. Histopathology. 2004;44(5):490–7. doi: 10.1111/j.1365-2559.2004.01880. [DOI] [PubMed] [Google Scholar]
- 16.Lehto M, Koivuluhta M, Wang G, Amghaiab I, Majuri ML, Savolainen K, et al. Epicutaneous natural rubber latex sensitization induces T helper 2-type dermatitis and strong prohevein-specific IgE response. J Invest Dermatol. 2003;120(4):633–40. doi: 10.1046/j.1523-1747.2003.12104.x. [DOI] [PubMed] [Google Scholar]
- 17.Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 18.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59(5):987–90. [PubMed] [Google Scholar]
- 19.van Rees BP, Saukkonen K, Ristimaki A, Polkowski W, Tytgat GN, Drillenburg P, et al. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196(2):171–9. doi: 10.1002/path.1033. [DOI] [PubMed] [Google Scholar]
- 20.Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, et al. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005;65(2):664–70. [PubMed] [Google Scholar]
- 21.Corcoran CA, He Q, Huang Y, Sheikh MS. Cyclooxygenase-2 interacts with p53 and interferes with p53-dependent transcription and apoptosis. Oncogene. 2005;24(9):1634–40. doi: 10.1038/sj.onc.1208353. [DOI] [PubMed] [Google Scholar]
- 22.Han JA, Kim JI, Ongusaha PP, Hwang DH, Ballou LR, Mahale A, et al. P53-mediated induction of Cox-2 counteracts p53- or genotoxic stress-induced apoptosis. Embo J. 2002;21(21):5635–44. doi: 10.1093/emboj/cdf591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo O, Schiavone N, Papucci L, Sardi I, Magnelli L, Franchi A, et al. Down-regulation of nitric oxide synthase-2 and cyclooxygenase-2 pathways by p53 in squamous cell carcinoma. Am J Pathol. 2003;163(2):723–32. doi: 10.1016/S0002-9440(10)63699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Moraes E, Dar NA, de Moura Gallo CV, Hainaut P. Cross-talks between cyclooxygenase-2 and tumor suppressor protein p53: Balancing life and death during inflammatory stress and carcinogenesis. Int J Cancer. 2007;121(5):929–37. doi: 10.1002/ijc.22899. [DOI] [PubMed] [Google Scholar]
- 25.Valente G, Ferrari L, Kerim S, Gervasio CF, Ricci E, Migliaretti G, et al. Evidence of p53 immunohistochemical overexpression in ethmoidal mucosa of woodworkers. Cancer Detect Prev. 2004;28(2):99–106. doi: 10.1016/j.cdp.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Bandoh N, Hayashi T, Kishibe K, Takahara M, Imada M, Nonaka S, et al. Prognostic value of p53 mutations, bax, and spontaneous apoptosis in maxillary sinus squamous cell carcinoma. Cancer. 2002;94(7):1968–80. doi: 10.1002/cncr.10388. [DOI] [PubMed] [Google Scholar]
- 27.Holmila R, Bornholdt J, Wolff H, Heikkilä P, Steiniche T, Dictor M, Schmaus A, Hansen J, Luce D, Wallin H, Husgafvel-Pursiainen K. Molecular changes in sino-nasal cancer related to wood dust exposure: TP53 mutations and COX-2 expression. Proc Am Ass Cancer Res AACR Ann Meeting 2007; 2007. [Google Scholar]
- 28.IARC. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 29.Kuper H, Boffetta P, Adami HO. Tobacco use and cancer causation: association by tumour type. J Intern Med. 2002;252(3):206–24. doi: 10.1046/j.1365-2796.2002.01022.x. [DOI] [PubMed] [Google Scholar]


