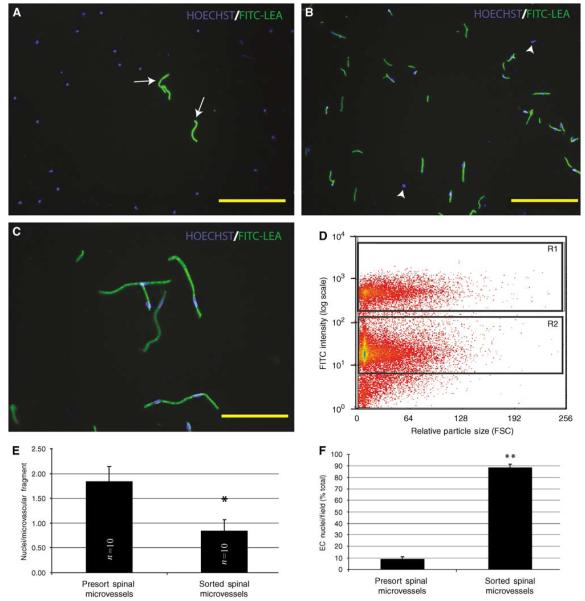
Figure 2.
Fluorescence-activated cell sorter isolation of FITC-LEA-infused spinal microvascular fragments results in a highly enriched population of smvECs. In presort spinal cord homogenates prepared from spinal tissue of FITC-LEA-infused mice, Hoechst-labeled EC nuclei account for a minority of cellular profiles (A; arrows). After FACS isolation of FITC-LEA-bound smvECs, few nuclei are present that are not intimately associated with microvascular fragments (B; arrowheads). Immediately after isolation, the microvascular fragments maintain their characteristic morphologic features with very few nonassociated nuclei apparent (C). A representative FACS plot (D) shows the labeled smvECs showing fluorescence approximately one order of magnitude greater than the background associated with the sample and clearly separating into the selected gate (R1) that contains the smvEC isolation. Quantitative analysis indicates that isolated smvECs have significantly fewer nuclei per FITC-LEA-bound microvascular fragment of the average nuclei per fragment (E) illustrates that smvECs experience significant shear stress as they are collected. Microscopic analyses of FACS-isolated smvEC collections show that approximately 90% of nuclei are associated with FITC-LEA microvascular fragments, a significant increase compared with total presort spinal homogenates. Quantitative assessment of microvascular fragment isolation is expressed as mean±s.d. (n = 10 per experimental group) *sorted is significantly different from presort (d.f. = 16; P = 4.04 × 10−7) and **sorted is significantly different from presort (d.f. = 14; P = 1.32 × 10−18). Scale bars = 150 μm (A, B); 50 μm (C).