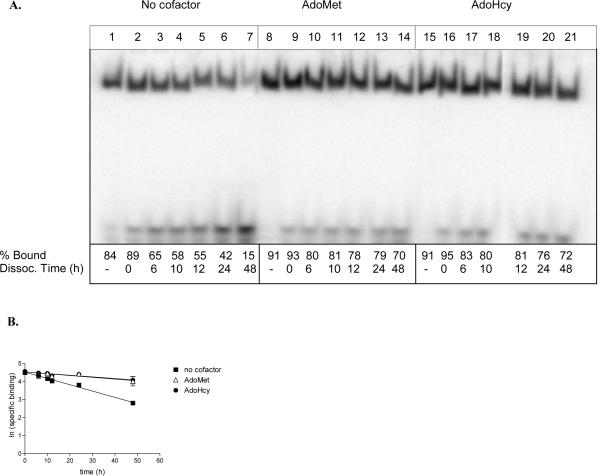
Figure 5.
Dissociation of 2P-ODN from M.HhaI. (A) Binding reactions containing 45 nM 32P-end labeled 2P-ODN, 108 nM M.HhaI, 75 ng poly dAdT:dAdT, and 100 μM cofactor (as indicated) were incubated at 22 °C for 30 min. A 100-fold excess unlabeled 2P-ODN was added to all reactions with the exception of those in lanes 1, 8, and 15, and the incubation continued for the indicated times. Zero time points (lanes 2, 9, and 16) represent reactions in which the unlabeled ODN was added immediately before loading onto a non-denaturing polyacrylamide gel. The percent bound ODN (% bound) was determined by imaging of the dried gel on a Typhoon PhosphoImager (Amersham). (% bound = [counts in enzyme-ODN complex/counts in enzyme-ODN complex + counts in free ODN] X 100). (B) The off rate was calculated by plotting the ln (specific binding) versus the time of incubation in the presence of cold competitor. A summary of the data is presented in Table 1.