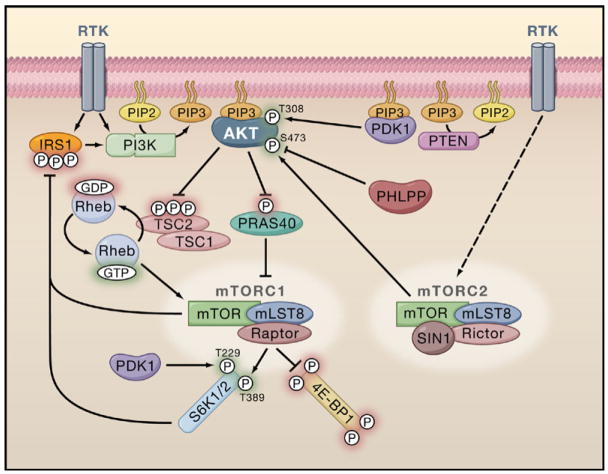
Figure 1. Upstream Activation of Akt by Growth Factors.
Also depicted is the complex relationship between Akt signaling and mTOR. Activated receptor tyrosine kinases (RTKs) activate class I phosphatidylinositol 3-kinase (PI3K) through direct binding or through tyrosine phosphorylation of scaffolding adaptors, such as IRS1, which then bind and activate PI3K. PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3), in a reaction that can be reversed by the PIP3 phosphatase PTEN. AKT and PDK1 bind to PIP3 at the plasma membrane, and PDK1 phosphorylates the activation loop of AKT at T308. RTK signaling also activates mTOR complex 2 (mTORC2) through a currently unknown mechanism, and mTORC2 phosphorylates Akt on the hydrophobic motif S473, which can be dephosphorylated by the S473 phosphatase PHLPP. Akt activates mTOR complex 1 (mTORC1) through multisite phosphorylation of TSC2 within the TSC1-TSC2 complex, and this blocks the ability of TSC2 to act as a GTPase-activating protein (GAP) for Rheb, thereby allowing Rheb-GTP to accumulate. Rheb-GTP activates mTORC1, which phosphorylates downstream targets such as 4E-BP1 and the hydrophobic motif on the S6 kinases (S6Ks; T389 on S6K1). PDK1 phosphorylates the activation loop on the S6Ks (T229 on S6K1) in a reaction independent of PDK1 binding to PIP3. Akt can also activate mTORC1 by phosphorylating PRAS40, thereby relieving the PRAS40-mediated inhibition of mTORC1. Once active, both mTORC1 and S6K can phosphorylate serine residues on IRS1, which targets IRS1 for degradation, and this serves as a negative feedback mechanism to attenuate PI3K-Akt signaling. See text for references to recent reviews detailing Akt regulation and mTOR signaling.