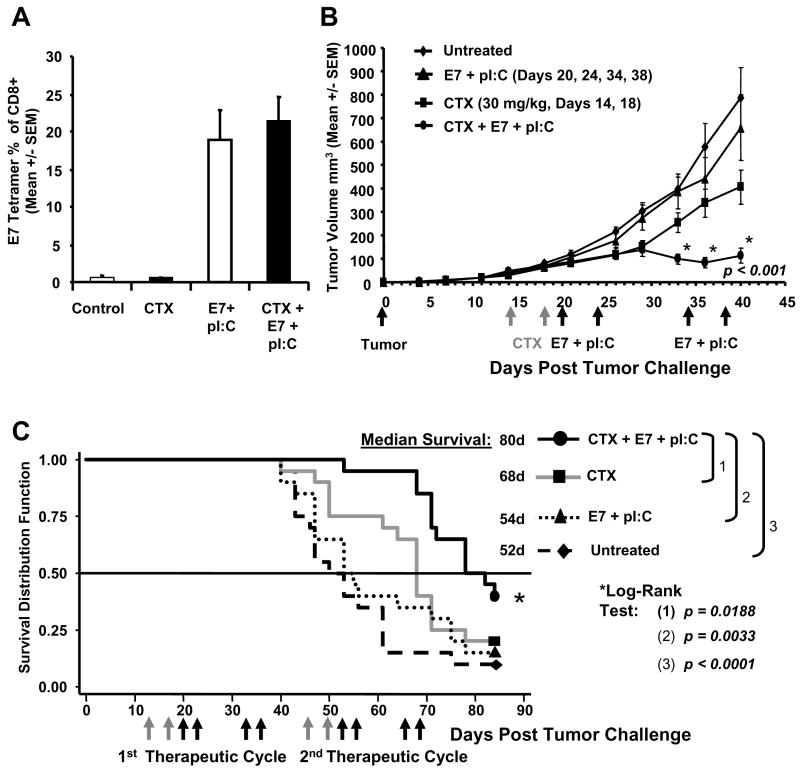
Fig. 4.
Adjunctive therapy with CTX enables immunotherapy in a more advanced disease setting. Mice were inoculated with 105 HPV-16 transformed C3.43 tumor cells on day 0, treated with CTX on Day 14 and 18 (n=20), immunized with E7 49-57 HPV peptide and pI:C in bilateral inguinal lymph nodes on day 20, 24, 34, and 38 (n=20), or treated with CTX then E7 + pI:C immunotherapy (n=20). Immune response (A) and tumor progression (B) was compared to untreated tumor control mice (n=20). The immune response following immunotherapy was measured by E7 49-57 tetramer staining on day 45 from peripheral blood. The immunized only group (E7 + pI:C) displayed HPV specific immune responses in the range of 20% with no observed inhibition of immune response in animals treated with CTX + E7 + pI:C which generated a similar tetramer response. The naïve control (n=5) and CTX control groups generated background levels of tetramer staining (A). CTX + E7 + pI:C induced significant tumor regression (p < 0.001) compared to E7 + pI:C immunotherapy alone, CTX alone, and untreated tumor controls (B). Evaluation of adjunctive therapy on animal survival (C). A second therapeutic cycle was administered with animals receiving CTX on day 46 and 50 (n=20), lymph node immunization with E7 + pI:C on day 52, 56, 65, and 69 (n=20), or treated with CTX + E7 + pI:C (n=20). Grey arrows indicate CTX treatment and black arrows indicate immunization days. Log-Rank statistical tests confirmed that survival in the CTX + E7 + pI:C group was significantly longer than survival in the control group (p < 0.0001, n=20), the CTX only group (p = 0.0188) and the E7 + pI:C immunotherapy only group (p = 0.0033).