Abstract
Conditioned taste aversion (CTA) is a form of associative learning in which the pairing of a taste with a toxin causes an animal to avoid the taste. NMDA receptor mediated neurotransmission has been implicated in CTA, but the role of the NMDA receptor glycine-binding site has not been examined. To examine the effects on CTA of the glycinergic NMDA receptor agonist D-cycloserine, rats received D-cycloserine (15 mg/kg, i.p.) or vehicle 15 min before 10-min access to 0.125% saccharin, followed by a low dose of LiCl (19 mg/kg, i.p.). CTA was measured with 24-h, 2-bottle preference tests between water and saccharin. Vehicle-treated rats formed a mild CTA that rapidly extinguished, while D-cycloserine-treated rats formed a stronger CTA that extinguished slowly. The effect of D-cycloserine was specific to the NMDA receptor glycine-binding site, because pretreatment with HA-966 (6 mg/kg), a partial glycinergic agonist, blocked enhancement by D-cycloserine. Three follow-up experiments suggest that the enhancement of CTA was not due to an aversive effect of D-cycloserine. First, saccharin paired with D-cycloserine (15 mg/kg) alone did not induce a CTA, although a higher dose (30 mg/kg) did significantly lower saccharin preference. Second, pretreatment with D-cycloserine did not increase the duration of “lying-on-belly” behavior induced by LiCl. Third, pretreatment with D-cycloserine did not increase c-Fos induction by either LiCl or vehicle injection in central visceral relays (the nucleus of the solitary tract, the parabrachial nucleus, the central nucleus of the amygdala, the supraoptic nucleus, and the paraventricular nucleus). These results confirm the participation of NMDA receptor, and specifically the glycine-binding site of NMDA receptor, in CTA learning.
Keywords: NMDA receptor, glycine, D-serine, saccharin, lithium, lying-on-belly, c-Fos
Introduction
Conditioned taste aversion (CTA) is a form of associative learning in which animals avoid the taste or flavor of a food (e.g. the sweet taste of saccharin) that has been paired with a toxic or aversive postingestive consequence (e.g. a systemic injection of LiCl). As in many other forms of learning and memory in rodents, ionotropic glutamate receptors of the N-methyl-D-aspartate NMDA subtype have been implicated in CTA learning. Antagonists at the NMDA receptor have been shown to block or attenuate CTA learning. In particular, competitive antagonists (e.g. APV or CPP) block CTA learning when injected into the gustatory cortex before or around the time of CTA acquisition (Berman et al., 2000; Escobar et al., 2002; Escobar et al., 1998; Ferreira et al., 2002; Gutierrez et al., 1999; Rosenblum et al., 1997). In addition, the 2B subunit of the NMDA receptor in the gustatory cortex undergoes tyrosine phosphorylation when rats drink a novel taste solution (Rosenblum et al., 1997), suggesting that posttranslational modification of NMDA receptor may also be important during the association of a novel taste and a toxic effect. Although the amygdala is also a critical site for CTA function, blockade of NMDA receptor in the amygdala does not attenuate CTA learning as consistently as blockade in the gustatory cortex (Ferry and Di Scala, 2000; Hatfield and Gallagher, 1995; Tucci et al., 1998; Yasoshima et al., 2000).
The NMDA receptor is activated by the presence of endogenous glutamate, postsynaptic depolarization that removes the Mg++ block, and occupancy of the NMDA receptor glycine-binding site (Johnson and Ascher, 1987). Although it was once thought that endogenous levels of glycine were sufficient to saturate the NMDA receptor glycine-binding site under most conditions (Johnson and Ascher, 1987), evidence has accumulated that low levels of a ligand may constrain NMDA receptor neurotransmission at many synapses (Danysz and Parsons, 1998). Thus, activity at the glycine-binding site can be negatively regulated by the removal of glycine by synaptic transporters (Chen et al., 2002), or positively regulated by the production of endogenous ligands, such as D-serine which is synthesized and released by perisynaptic astrocytes (Schell et al., 1995; Wolosker et al., 1999).
A consequence of limited endogenous activity at the NMDA receptor glycine-binding site is that exogenous glycine agonists may increase submaximal NMDA receptor neurotransmission and thus enhance NMDA receptor-dependent processes, including learning. D-cycloserine is a high affinity NMDA receptor glycine-binding site agonist that is rapidly transported into the central nervous system after systemic injection (Baran et al., 1995). Systemic administration of D-cycloserine has been shown to enhance learning in several animal models, including trace eye-blink conditioning (Thompson et al., 1992), spatial learning in the water-maze (Temple and Hamm, 1996), inhibitory avoidance (Land and Riccio, 1999), and extinction of fear conditioning as measured with freezing (Richardson et al., 2004) or with fear-potentiated startle (Walker et al., 2002). More recently, D-cycloserine has been shown to accelerate extinction of phobias in humans when administered prior to desensitization exposure sessions (Ressler et al., 2004).
Here we demonstrate that 1. D-cycloserine enhances CTA induced by pairing intake of saccharin with a low dose of LiCl. 2. D-cycloserine enhancement is blocked by pretreatment with HA-966, a partial agonist at the NMDA receptor glycine-binding site and 3. The enhancement of CTA is not due to an aversive effect of D-cycloserine alone, because low doses of D-cycloserine itself did not induce a CTA, nor did D-cycloserine enhance LiCl-induced “lying-on-belly” behavior or central c-Fos expression. These results suggest that D-cycloserine is acting specifically at the NMDA receptor to enhance CTA learning.
Materials and Methods
Subjects
Male Sprague-Dawley rats (200-350 grams; Charles River) were housed individually in polycarbonate cages in a temperature-controlled colony room at the Biomedical Research Facility at the Florida State University. The light/dark cycle was 12:12 with lights on at 0700 hours. All conditioning trials were conducted during the light cycle. The rats had free access to pelleted Purina Rat Chow 5001 and water ad libitum from one of two available spouts except when specified otherwise. The experiments and all procedures were approved by the Florida State University Institutional Animal Use and Care Committee.
Conditioning Procedure
Seven days before the conditioning day, rats were placed on a water restriction schedule under which they received daily water access in one drinking session. The initial session was three hours in length and the session times were diminished each day so that the day before conditioning the rats received their daily water access in a single 10-minute session. Rats were weighed on the day before conditioning.
On conditioning day, the rats received intraperitoneal injections (i.p.) of D-cycloserine (Sigma RBI, St. Louis MO), HA-966 ([+/-]-3-amino-1-hydroxy-2-pyrrolidone; Sigma RBI, St. Louis MO), or vehicle (0.15 M NaCl or water, respectively) as a pretreatment before taste aversion conditioning as described in the individual experiments below. To induce CTA, the rats were allowed 10-min access to 0.125% sodium saccharin (saccharin) as the conditioned stimulus (CS). Twenty minutes after the end of saccharin access, a relatively low dose of LiCl (38 mM, 12 ml/kg i.p., made isotonic with NaCl) was administered as the unconditioned stimulus (US). This dose of LiCl was chosen because it induces a significant but submaximal CTA, thus allowing a D-cycloserine-induced increase to be seen (Nachman and Ashe, 1973). Control rats received NaCl (0.15M, 12 ml/kg i.p.) as a non-aversive US. Three hours after LiCl injection, rats were given ad libitum water access overnight.
Preference Tests
In order to test for an increase in CTA magnitude or resistance to extinction, a series of 24-h 2-bottle preference tests were initiated on the day after conditioning. Rats were given access to two bottles, one containing the saccharin and one containing water. The left-right position of the two bottles was reversed every day in order to observe side preferences. Fluid consumption was measured every 24 h by weighing the drinking bottles to the nearest 0.1g. A preference score was calculated as a ratio of saccharin and total fluid consumption in the following manner:
The preference tests were continued for 4-13 post-conditioning days. Because saccharin access during the preference tests was not paired with any aversive stimulus, the preference tests constituted extinction trials. A CTA was considered extinguished when the average saccharin preference of LiCl rats was not significantly different (p > 0.05) from the average preference of control rats receiving 0.15M NaCl as a US.
Statistics
The first day of 2-bottle preference tests was taken as a measure of the initial magnitude of CTA. Four to 13 days of consecutive 2-bottle preference test days were taken as a measure of extinction rate. Significant effects were detected by 1- or 2-way ANOVA (with test day as a repeated measure); posthoc comparisons were made with the Newman-Keuls multiple comparison test.
Experiment 1
A total of 24 rats were housed and placed on a water restriction as described above. After 7 days of diminished water access, the rats were divided into 3 groups (n=6 per group):
A “DCS/vehicle” group, that was injected with D-cycloserine (15 mg/kg, i.p), and 15 min later given 10 min access to saccharin, and 20 minutes after the end of saccharin access were injected with NaCl (0.15M, 12 ml/kg). This group served as a control group that was not expected to acquire a CTA, and thus show a high preference for saccharin during subsequent 2-bottle preference testes.
A “vehicle/LiCl” group, that was injected with NaCl (0.15 M, 1 ml/kg, i.p) 15 min prior to 10-min access to saccharin, and 20 minutes after the end of saccharin access were injected with LiCl (38 mM, 12ml/kg i.p.). Because this group consumed saccharin paired with a low dose of LiCl, the rats were expected to form a moderate but significant CTA.
A “DCS/LiCl” group, that was injected with D-cycloserine (15 mg/kg, i.p.) 15 min prior to 10-min access to saccharin, and 20 minutes after the end of saccharin access were injected with LiCl (38 mM, 12ml/kg i.p.). This group was designed to test the effect of D-cycloserine pretreatment on a LiCl-induced CTA.
Three hours after conditioning, rats received ad libitum access to water overnight. The next day 24-h, 2-bottle preference tests were started and continued for 9 days.
Experiment 2
D-cycloserine increases neurotransmission at the NMDA receptor by acting as a high-affinity agonist of the glycine-binding site on the NR1 receptor subunit (Danysz and Parsons, 1998). Thus, blockade of the glycine-binding site can serve as a test of the specificity of D-cycloserine in enhancing CTA via the NMDA receptor: if a glycine antagonist counteracts the effects of D-cycloserine, the facilitation is likely mediated via the NMDA receptor. Conversely, if a glycine antagonist fails to block its effects, then D-cycloserine may have a non-specific effect. Therefore, we tested the role of the glycine-binding site by administering HA-966, a partial agonist at the glycine-binding site, prior to D-cycloserine injection and CTA acquisition. As a partial agonist, HA-966 effectively blocks D-cycloserine agonist activity (Moraes Ferreira and Morato, 1997; Myhrer, 1994; Walker et al., 2002).
A total of 36 rats were individually housed and placed on a water restriction schedule as described above. On conditioning day, all rats received 3 injections (2 before and 1 after 10-min access to saccharin): the first injection was given 30 min before saccharin access, the second injection 15 min before saccharin access, and the third injection 20 min after the end of saccharin access. The rats were divided into 5 groups:
A “vehicle control” group (n=6) that was first injected with water (1 ml/kg, i.p.), then 15 min later injected with NaCl (0.15 M, 1 ml/kg), then injected again 20 min after saccharin access with NaCl (0.15M, 12 ml/kg). This group served as a control for repeated injection, but was not expected to acquire a CTA against saccharin.
A “vehicle/LiCl control” group (n=12) that was first injected with water (1 ml/kg, i.p.), then 15 min later injected with NaCl (0.15 M, 1 ml/kg), then injected 20 min after saccharin access with LiCl (38 mM, 12 ml/kg). Because this group received saccharin paired with a low dose of LiCl, it was expected to acquire a moderate CTA.
A “HA-966/LiCl” group (n=6) that was first injected with HA-966 (6 mg/kg i.p.), then 15 min later injected with NaCl (0.15 M, 1 ml/kg), then injected 20 min after saccharin access with LiCl (38 mM, 12 ml/kg). This group was designed to determine the effect of HA-966 pretreatment on LiCl-induced CTA, and in particular to establish that HA-966 by itself did not diminish LiCl-induced CTA.
A “DCS/LiCl” group (n=6) that was first injected with water (1 ml/kg, i.p.), then 15 min later injected with D-cycloserine (15 mg/kg, i.p.), then injected 20 min after saccharin access with LiCl (38 mM, 12 ml/kg, i.p.). This group was designed to replicate the enhancement by D-cycloserine of LiCl-induced CTA, as seen in Experiment 1.
A “HA-966/DCS/LiCl” group (n=6) that was first injected with HA-966 (6 mg/kg i.p.), then 15 min later injected with D-cycloserine (15 mg/kg, i.p.), then injected 20 min after saccharin access with LiCl (38 mM, 12 ml/kg, i.p.). This group was designed to determine the effect of HA-966 pretreatment on the enhancement of LiCl-induced CTA by D-cycloserine.
Three hours later, rats received ad libitum access to water overnight. The next day 24-h, 2-bottle preference tests were started and continued for 13 days.
Experiment 3
If a drug has toxic or non-specific aversive effects in rats, then enhancement of a CTA by the drug might be the trivial result of the summation of the drug's aversive effects and LiCl's aversive effects. Therefore, Experiment 3 was designed to determine if D-cycloserine alone was sufficient to induce a CTA. In order to parallel the pretreatment in Experiments 1 and 2, D-cycloserine as the US was administered prior to CS access (i.e. backwards conditioning).
A total of 24 rats were placed on a water restriction schedule as above. After 7 days of diminished water access, rats were injected with one of 4 doses of D-cycloserine (0, 7.5, 15, or 30 mg/kg i.p.; n = 6 per group). Fifteen minutes after D-cycloserine administration, rats were given 10-min access to saccharin. Three hours later, rats received ad libitum access to water overnight. The next day 24-h, 2-bottle preference tests were started and continued for 4 days.
Experiment 4
The effect of D-cycloserine pre-treatment on LiCl-induced malaise was also assessed by observing the frequency and duration of “lying-on-belly” behavior. Within the first hour after systemic injection of high doses of LiCl, rats will spontaneously lie practically motionless with their abdomens pressed against the floor of their cages (Meachum and Bernstein, 1990). This “lying-on-belly” posture is very distinct from their normal resting or sleeping posture. Quantification of “lying-on-belly” has been used to assess the aversive effects of LiCl (Bernstein et al., 1992; Meachum and Bernstein, 1992; Navarro and Cubero, 2003; Stafstrom-Davis et al., 2001). If D-cycloserine was enhancing the presumed malaise induced by LiCl, then it might also increase the incidence of “lying-on-belly” induced by a low dose of LiCl.
Rats (n = 8) were injected with D-cycloserine (15 mg/kg, i.p.) or vehicle (0.15 M NaCl, 1 ml/kg, i.p.) and individually placed for observation in a large polypropylene cage (37 cm wide by 47 cm long by 20 cm high) with woodchip bedding. Fifteen minutes after D-cycloserine or vehicle injection, all rats were injected with LiCl (38 mM, made isotonic with NaCl, 12 ml/kg, i.p.). Rats were observed for 30 minutes after the LiCl injection; the onset and duration of “lying-on-belly” was recorded for each rat. As a positive control, rats were also observed for 30 min after receiving an injection of a higher dose of LiCl (0.15M, 12 ml/kg i.p.). All rats received all three treatments, with a minimum of 72 hours between treatments.
Experiment 5
As a third measure of enhancement by D-cycloserine of LiCl-induced malaise, c-Fos induction was examined. It is well-established that systemic injection of LiCl induces c-Fos in visceral and neuroendocrine relays of the rat brain (Houpt et al., 1994; Koehnle and Rinaman, 2007; Lamprecht and Dudai, 1995; Spencer and Houpt, 2001; Swank and Bernstein, 1994; Yamamoto et al., 1992), such as the nucleus of the solitary tract (NTS), the lateral parabrachial nucleus (PBN), the central nucleus of the amygdala (CeA), and the hypothalamic supraoptic (SON) and paraventricular nuclei (PVN). If D-cycloserine enhanced the central response to LiCl per se, then it might also increase levels of neuronal activation observed as c-Fos expression.
Rats (n = 24) were injected with either D-cycloserine (15 mg/kg, i.p.) or vehicle (0.15 M NaCl, 1 ml/kg, i.p.). Fifteen minutes after D-cycloserine or vehicle injection, half of the rats in each pre-treatment group were injected with either LiCl (38 mM, made isotonic with NaCl, 12 ml/kg, i.p.) or NaCl (0.15M, 12 ml/kg, i.p.). Thus there were four groups (n=6/group): NaCl/NaCl, NaCl/LiCl, DCS/NaCl, and DCS/LiCl. One hour after the second injection, rats were overdosed with sodium pentobarbital and transcardially perfused with saline followed by phosphate-buffered 4% formaldehyde as previously described (Houpt et al., 1994). The brains were removed and cryoprotected in 30% sucrose. Forty micron coronal sections were cut on a freezing, sliding microtome. Alternate sections were collected from the medulla at the level of the NTS (bregma − 13.24 mm to −14.08 mm) and the pons at the level of the PBN (bregma −9.16 mm to − 9.68 mm). Every fourth section was collected from the forebrain through the hypothalamus and amygdala (bregma −1.8 mm to −3.3 mm). Coordinates were based on Paxinos and Watson's atlas (Paxinos and Watson, 1986).
Sections were immediately processed for c-Fos-like immunoreactivity. Free-floating tissue sections were washed twice for 15 min in 0.1 M phosphate-buffered saline (PBS) and then incubated for 30 min in 0.2% Triton/ 1% bovine serum albumin (BSA)/PBS. After two washes in PBS/BSA for 15 min each, sections were incubated overnight with a rabbit anti-c-Fos antiserum (Ab-5, Oncogene Research) at a dilution of 1:20,000. After two 15 min washes in PBS/BSA, sections were then incubated for 1 h with a biotinylated goat anti-rabbit antibody (Vector Laboratories) at a dilution of 1:200. Antibody complexes were amplified using the Elite Vectastain ABC kit (Vector Laboratories), and visualized by a 5-min reaction in 0.05% 3,3-diaminobenzidine tetrahydrochloride (DAB). Sections were stored in 0.1 M PB until mounted onto gelatin-coated glass slides, counterstained with methyl green, and coverslipped using Permount.
Cells expressing darkly-positive, nuclear c-FLI were quantified using a custom software program (MindsEye, T. Houpt). Regions were digitally-captured at 40× magnification. Counting was restricted to the regions of interest delineated by a hand-drawn outline. Bilateral cell counts were averaged for 3 sections of each brain region for each rat. The individual mean counts for each region were then averaged across rats within experimental groups.
Results
Experiment 1: D-cycloserine and LiCl-induced conditioned taste aversion
On the first day of 2-bottle preference testing, ANOVA revealed a significant effect of treatment [F (2,21) = 10.2, p<0.001]. All LiCl-treated rats showed a decreased preference compared to DCS/vehicle rats. The preference of DCS/LiCl rats that had been pretreated with 15 mg/kg D-cycloserine was not significantly lower (p = 0.08) than the preference of vehicle/LiCl rats on the first day (see Figure 1A).
Figure 1.

Pretreatment with D-cycloserine increased the initial magnitude of saccharin preference as seen after one 24-h, 2-bottle preference test (A) and prolonged expression across 9 extinction days of 2-bottle preference tests (B). Rats receiving D-cycloserine (15 mg/kg) before saccharin-vehicle pairing (DCS/vehicle group) showed a high saccharin preference on all days. Rats receiving saccharin paired with LiCl alone (vehicle/LiCl group) showed a moderate CTA with significantly lower preferences than DCS/vehicle rats on all 9 test days. Compared vehicle/LiCl, the combination of D-cycloserine (15 mg/kg) and LiCl significantly enhanced CTA expression on all 9 days (DCS/LiCl group). * p < 0.05 vs. DCS/vehicle; † p < 0.05 vs. vehicle/LiCl.
Across 9 days of 2-bottle preference tests, 2-way ANOVA revealed an effect of treatment [F (2,21) = 13.9, p < 0.0005] and day [F (8,168) = 5.6, p< 0.0001] but no interaction (see Figure 1B). Post-hoc comparisons showed significant differences among the 3 groups. DCS/vehicle control rats maintained a high intake of saccharin to the near exclusion of water intake. Compared to the controls, vehicle/LiCl rats showed a significantly decreased preference for saccharin that persisted for 8 of 9 days. Rats pretreated with 15 mg/kg D-cycloserine prior to the pairing of saccharin and LiCl showed a CTA that was significantly greater than that of vehicle/LiCl rats across 7 of the 9 days of 2-bottle preference testing, without extinguishing.
Experiment 2: Effect of HA-966 pretreatment on D-cycloserine and CTA
On the first day of 2-bottle testing, ANOVA showed a significant effect of treatment [F (4,35) = 5.46, p < 0.005; see Figure 2A]. All LiCl-treated groups showed a significantly decreased preference for saccharin compared to the vehicle control group. The preference of the HA-966/LiCl group was not different from the vehicle/LiCl group. Of the two D-cycloserine-treated groups, DCS/LiCl rats had a significantly greater CTA compared to vehicle/LiCl rats; the first-day magnitude of CTA in HA-966/DCS/LiCl rats was not different from vehicle/LiCl rats, however.
Figure 2.
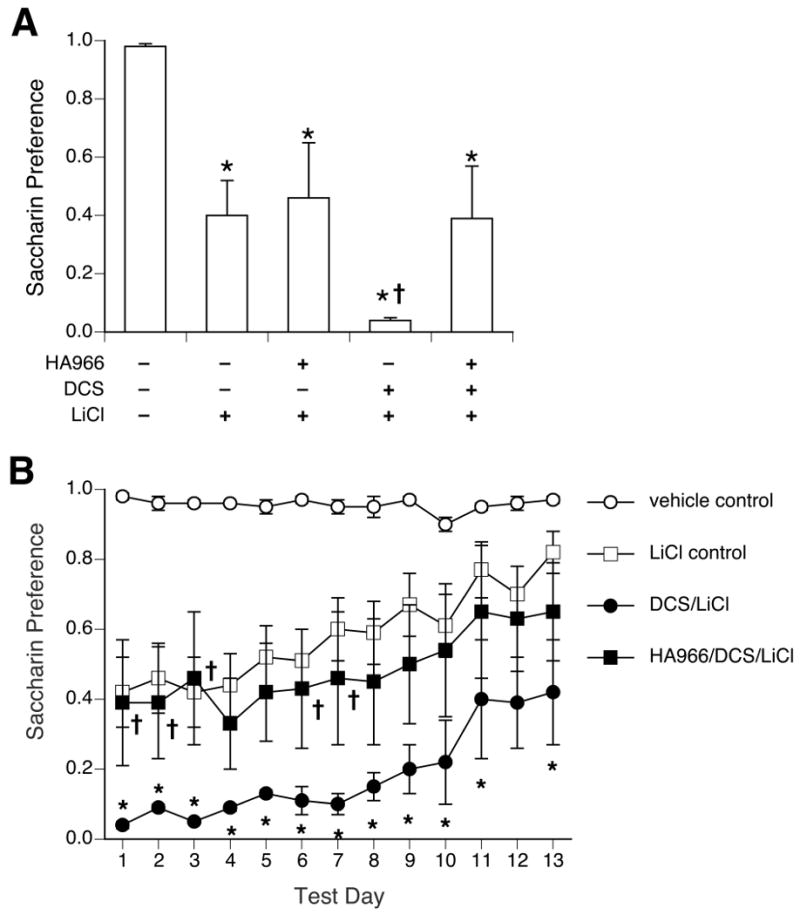
Pretreatment with HA-966 blocked the effect of D-cycloserine on the initial magnitude of CTA as seen after one 24-h, 2-bottle preference test (A) and on extinction across 13 days of 2-bottle preference tests (B). All groups that received saccharin paired with LiCl showed a significant CTA compared to vehicle control rats for at least the first 8 days of extinction. Rats in the DCS/LiCl group showed an enhanced CTA, with significantly lower preferences than LiCl control rats on almost all days. HA-966 did not alter the magnitude of the saccharin-LiCl CTA, but did block the enhancement by D-cycloserine (HA-966/DCS/LiCl preferences were not different from LiCl control on any day). * p < 0.05 vs. LiCl control; † p < 0.05 vs. DCS/LiCl.
A 2-way ANOVA comparing the HA-966/ LiCl and veh/ LiCl groups across extinction days showed no significant effect of treatment, and so these two groups were combined into a single “LiCl control” group (n= 18) for subsequent analysis. A 2-way ANOVA comparing treatment groups (vehicle control, LiCl control, DCS/LiCl, and HA-966/DCS/LiCl) across 13 days of 2-bottle preference testing showed a significant effect of treatment [F(3,32) = 7.2, p < 0.001] and extinction days [F(12,384) = 8.1, p < 0.001], but no interaction (see Figure 2B). While the vehicle control rats maintained a high preference for saccharin, all LiCl-treated groups showed decreased preferences for saccharin that slowly increased across days. Preference scores of the LiCl control group was significantly lower than the vehicle control group on days 1-8. Likewise, preference scores for the DCS/LiCl group and the HA-966/DCS/LiCl group were significantly lower than the vehicle control group across all 13 days. Pretreatment with D-cycloserine alone (DCS/LiCl group) significantly lowered the preference scores of LiCl-injected rats on all days except day 12. However, pretreatment with HA-966 prior to D-cycloserine reversed this effect: the preference scores of the HA-966/DCS/LiCl group were not significantly different from LiCl control group on any day, and were significantly higher than the DCS/LiCl group on days 1,2,3,6 and 7.
Experiment 3: D-cycloserine and saccharin alone
On the first day of 2-bottle testing, ANOVA showed a significant effect of treatment [F (3,20) = 7.16, p < 0.005]. Compared to vehicle-treated rats, only rats treated with 30 mg/kg D-cycloserine showed a significantly reduced saccharin preference (see Figure 3A). By 2-way ANOVA, the effect of treatment persisted across 4 days of 2-bottle preference testing [F (3,20) = 3.63, p < 0.05]; there was no effect of days, and no interaction (see Figure 3B).
Figure 3.

D-cycloserine induced CTA only at a high dose. Following pretreatment with D-cycloserine (0, 7 or 15 mg/kg), a high preference for saccharin was seen on the first day (A) and all 4 days of 24-h, 2-bottle preference testing (B). After pretreatment with D-cycloserine (30 mg/kg), rats showed a significantly decreased preference on all 4 test days as compared to vehicle treated rats. *p < 0.05 vs. vehicle-treated rats.
Experiment 4: D-cycloserine and LiCl-induced “lying-on-belly”
The low dose of LiCl (38 mM, 12 ml/kg) induced low levels of “lying-on-belly” behavior after vehicle or D-cycloserine pre-treatment. By paired t-test, there was no significant difference between the average time of onset (vehicle, 28.3 ± 1.1 min vs D-cycloserine, 24.3 ± 2.3, p > 0.05) or duration (vehicle: 0.9 ± 0.7 min, D-cycloserine: 2.6 ± 1.1 min, p > 0.05). In contrast, when the the higher dose of LiCl (0.15M, 12 ml/kg) was compared to the two other treatments by one-way ANOVA, there was a significant effect of treatment on both onset (F[2,22] = 35.1, p < 0.0001) and duration (F[2,22] = 33.3, p < 0.0001). The higher dose of LiCl induced “lying-on-belly” with a significantly shorter time to onset (11.4 ± 0.9 min) and significantly longer duration (11.9 ± 1.3 min) than the other two treatments (p < 0.0001 by Newman-Keuls post-hoc comparison).
Experiment 5: D-cycloserine and LiCl-induced c-Fos expression
Two-way ANOVAs with pretreatment (vehicle or D-cycloserine) and LiCl condition (LiCl or NaCl) as factors revealed a significant effect of LiCl injection, but not pretreatment, for the NTS [F(1,23)=9.1, p <0.01], PBN [F(1,23)=9.2, p <0.01], and CeA [F(1,23)=11.6, p <0.005]. There was no significant effect of either factor for the SON and PVN. Because there was no significant effect of D-cycloserine pretreatment, D-cycloserine- and vehicle-pretreated groups were combined and all LiCl-injected rats were compared to all NaCl-injected rats by t-test, which revealed significantly more c-Fos-positive cells in the NTS, PBN, CeA, and SON (but not the PVN) of LiCl-injected rats (see Figure 4).
Figure 4.
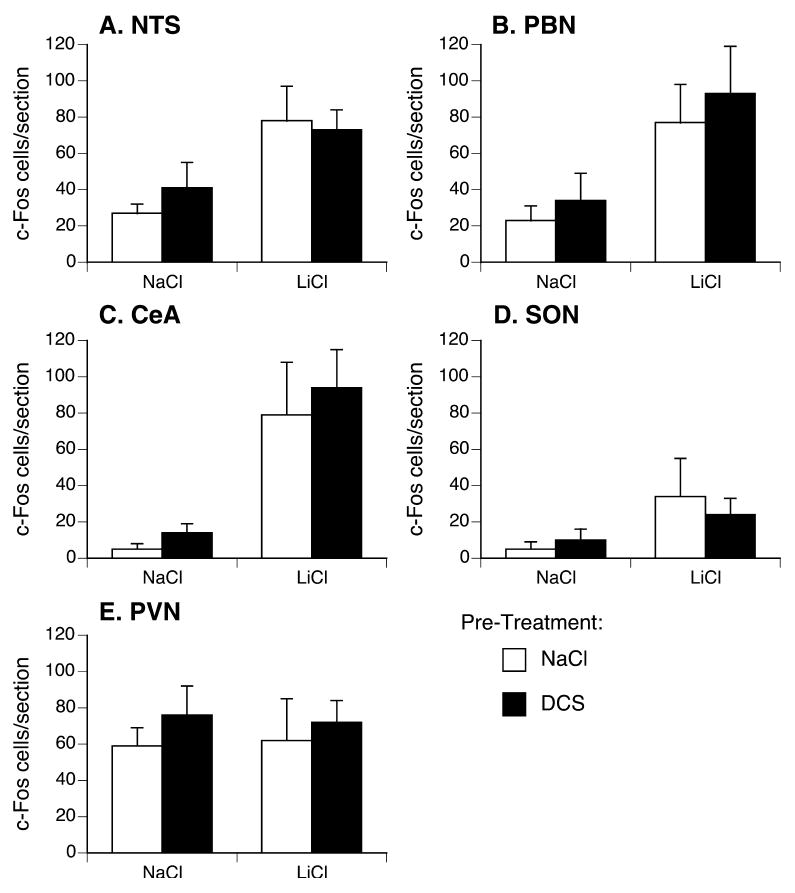
D-cycloserine pretreatment did not increase LiCl-induced c-Fos. Rats were pretreated with D-cycloserine (15 mg/kg, black bars) or vehicle (white bars). One hour after injection of NaCl (0.15M, 12ml/kg; left bars) or LiCl (38 mM, 12 ml/kg; right bars), brains were processed for c-Fos immunoreactivity. LiCl-induced significantly more c-Fos-positive cells in the NTS, PBN, CeA, and SON (but not PVN). There was no effect of D-cycloserine pretreatment on c-Fos-positive cells after either NaCl or LiCl injection in any brain region.
Discussion
Systemic administration of D-cycloserine 15 min before the pairing of saccharin intake with LiCl injection caused an enhancement of CTA learning as measured in subsequent 2-bottle preference tests. D-cycloserine induced a greater and more persistent CTA that did not extinguish within the 9-13 test days examined here. Pretreatment with the partial glycine agonist HA-966 blocked the effect of D-cycloserine on CTA learning, suggesting that D-cycloserine was acting specifically at the glycine-binding site of the NMDA receptor.
Because CTA learning is mediated by aversive, toxic, or malaise-inducing properties of a US such as LiCl, it is possible that the increased CTA observed with the combination of D-cycloserine and LiCl was due to aversive properties of D-cycloserine, or an enhancement by D-cycloserine of LiCl's aversive effects. Experiments 3, 4 and 5 were designed to determine if D-cycloserine was aversive or enhanced the aversive properties of LiCl. In Experiment 3, pretreatment with D-cycloserine by itself at 15 mg/kg and below was not sufficient to induce a CTA to saccharin. A higher dose of D-cycloserine (30 mg/kg) was sufficient to induce a CTA; given the widespread distribution of NMDA receptors both centrally and peripherally, it is perhaps not surprising that D-cycloserine should ultimately have an aversive effect. This is a common property of a wide variety of drugs (Gamzu, 1977).
In addition, Experiment 4 found that at the same doses at which D-cycloserine enhanced LiCl-induced CTA, D-cycloserine did not increase the incidence of LiCl-induced “lying-on-belly”. Quantification of “lying-on-belly” has been used to assess the aversive effects of LiCl (Bernstein et al., 1992; Meachum and Bernstein, 1992; Navarro and Cubero, 2003; Stafstrom-Davis et al., 2001).
In experiment 5, rats were treated with D-cycloserine and LiCl and the induction of the c-Fos immediate early gene was assessed in visceral relays of the rat brain (the NTS, PBN, CeA, SON, and PVN.) Compared to NaCl injections, the low-dose of LiCl induced significantly more c-Fos positive cells in the NTS, PBN, CeA, and SON. Pretreatment with D-cycloserine, however, had no effect on levels of c-Fos induction.
These two experiments demonstrate that D-cycloserine does not cause a simple summation of aversive visceral responses, as measured using a behavioral test and a marker of neuronal activation. While it is difficult to completely rule out an effect of D-cycloserine on transduction of the US, these results support the hypothesis that D-cycloserine is affecting the associative process more than the unconditioned response. Furthermore, we have reported elsewhere that D-cycloserine was able to enhance conditioned flavor-taste preference learning, which also suggests that D-cycloserine does not have a major aversive component (Golden and Houpt, 2005).
The ability of D-cycloserine to enhance CTA learning parallels the results of others for fear conditioning (Richardson et al., 2004), spatial learning (Temple and Hamm, 1996), the extinction of phobias (Ressler et al., 2004), and other models of learning. Importantly, D-cycloserine acted specifically at the glycine-binding site of the NMDA receptor to enhance CTA learning because HA-966, a partial glycine agonist, was able to block the enhancement of CTA by D-cycloserine. HA-966 has been used to demonstrate the specificity of D-cycloserine in other protocols, e.g. extinction of fear-potentiated startle (Walker et al., 2002). The enhancement of CTA by D-cycloserine also confirms a role for the NMDA receptor in CTA learning. Central NMDA receptors are required for full CTA learning, as demonstrated using NMDA receptor antagonists. For example, APV (a competitive antagonist) or CPP (a noncompetitive antagonist) administered into the gustatory cortex prior to or immediately after the pairing of saccharin and LiCl attenuates CTA acquisition (Berman et al., 2000; Escobar et al., 2002; Escobar et al., 1998; Ferreira et al., 2002; Gutierrez et al., 1999; Rosenblum et al., 1997).
NMDA receptor activation requires agonist binding to both the glycine- and glutamate-binding sites (Johnson and Ascher, 1987). The ability of D-cycloserine to enhance CTA learning suggests that sufficient glutamate is present, but that low levels of endogenous D-serine or glycine constrain NMDA receptor neurotransmission during taste and toxin association. Glycine, once thought to be widely distributed in the brain and present in synapses in high concentrations at most times, is now thought to be tightly regulated over time in restricted brain regions (Wolosker et al., 1999). Furthermore, D-serine has recently been proposed to be a wide-spread neuromodulater in the rat forebrain, where it is synthesized and released from glial cells into the synapse in response to neuronal stimulation (Schell et al., 1995). Because it is synthesized rather than released from presynaptic vesicles, synaptic concentrations of D-serine may lag behind glutamate levels and limit NMDA receptor neurotransmission.
Endogenous agonists at the glycine-binding site may also be necessary for normal CTA learning. The evidence collected with glutamatergic antagonists at the NMDA receptor such as APV demonstrates a necessary role for glutamate in CTA and thus implies a necessary co-agonist at the glycine-binding site. In other models of learning, glycinergic NMDA receptor antagonists have retarded learning (Bannerman et al., 1997; Biala and Kotlinska, 1999; Campbell et al., 1999; Kotlinska and Biala, 1999; Matsuoka and Aigner, 1996; Mead and Stephens, 1999; Ohno et al., 1994; Steele and Stewart, 1993; Watanabe Y, 1992). In this study, pre-treatment with the partial glycine agonist HA-966 alone did not cause any degradation of CTA learning, even though it blocked D-cycloserine-induced enhancement and can act as an antagonist when endogenous glycinergic tone is high (Henderson et al., 1990). While the affinity of systemically-administered HA-966 is sufficiently high and its potency sufficiently low to block exogenous treatments (Moraes Ferreira and Morato, 1997; Myhrer, 1994; Walker et al., 2002), more potent antagonists at the glycine-binding site (e.g. kynurenic acid) might be required to block low levels of endogenous glycinergic ligands (Henderson et al., 1990).
The sites at which D-cycloserine increased CTA learning cannot be determined from the current results, which used systemic injections. Among central sites, the gustatory cortex and amygdala are strong candidates. However, NMDA receptors are widely distributed throughout the hindbrain (e.g. nucleus of the solitary tract and parabrachial nucleus (Berthoud et al., 2001; Guthmann and Herbert, 1999)) and forebrain (Monyer et al., 1994). NMDA receptors are also expressed in the gut (Burns et al., 1994), which might contribute to visceral signals during CTA learning. While Experiment 5 shows that D-cycloserine does not increase LiCl-induced c-Fos in some brain regions, these results do not rule out the possibility that D-cycloserine enhances central neural activity induced by gustatory input, the toxic effects of LiCl, or the associative process itself that are not revealed by c-Fos expression. Future experiments could utilize site-specific injections of antagonists and visualize other markers of neural activity (e.g. MAP kinase) to localize the critical sites of glycinergic NMDA receptor neurotransmission in CTA learning.
Acknowledgments
Supported by National Institute for Deafness and Communicative Disorders DC03198 and an FSU Neuroscience Graduate Fellowship (R.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannerman DM, Butcher SP, Good MA, Morris RG. Intracerebroventricular infusion of the NMDA receptor-associated glycine site antagonist 7-chlorokynurenate impairs water maze performance but fails to block hippocampal long-term potentiation in vivo. Neurobiol Learn Mem. 1997;68:252–270. doi: 10.1006/nlme.1997.3797. [DOI] [PubMed] [Google Scholar]
- Baran H, Gramer M, Loscher W. Alterations in plasma and brain amino acids after administration of the glycine/NMDA receptor partial agonist, D-cycloserine, to mice and rats. Eur J Pharmacol. 1995;273:197–201. doi: 10.1016/0014-2999(94)00745-s. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1–2 and formation of a memory trace. J Neurosci. 2000;20:7017–7023. doi: 10.1523/JNEUROSCI.20-18-07017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IL, Chavez M, Allen D, Taylor EM. Area postrema mediation of physiological and behavioral effects of lithium chloride in the rat. Brain Res. 1992;575:132–137. doi: 10.1016/0006-8993(92)90432-9. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Earle T, Zheng H, Patterson LM, Phifer C. Food-related gastrointestinal signals activate caudal brainstem neurons expressing both NMDA and AMPA receptors. Brain Res. 2001;915:143–154. doi: 10.1016/s0006-8993(01)02826-8. [DOI] [PubMed] [Google Scholar]
- Biala G, Kotlinska J. Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol. 1999;34:175–182. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- Burns GA, Stephens KE, Benson JA. Expression of the mRNA for the N-methyl-D-aspartate (NMDAR1) receptor by the enteric neurons of the rat. Neurosci Lett. 1994;170:87–90. doi: 10.1016/0304-3940(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Butelman ER, Woods JH. Effects of (+)-HA-966, CGS-19755, phencyclidine, and dizocilpine on repeated acquisition of response chains in pigeons: systemic manipulation of central glycine sites. J Pharmacol Exp Ther. 1999;289:521–527. [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2002;89:691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons AC. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–566. [PubMed] [Google Scholar]
- Escobar ML, Alcocer I, Bermudez-Rattoni F. In vivo effects of intracortical administration of NMDA and metabotropic glutamate receptors antagonists on neocortical long-term potentiation and conditioned taste aversion. Behav Brain Res. 2002;129:101–106. doi: 10.1016/s0166-4328(01)00329-1. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Alcocer I, Chao V. The NMDA receptor antagonist CPP impairs conditioned taste aversion and insular cortex long-term potentiation in vivo. Brain Res. 1998;812:246–251. doi: 10.1016/s0006-8993(98)00931-7. [DOI] [PubMed] [Google Scholar]
- Ferreira G, Gutierrez R, De la Cruz V, Bermudez-Rattoni F. Differential involvement of cortical muscarinic and NMDA receptors in short- and long-term taste aversion memory. Eur J Neurosci. 2002;16:1139–1145. doi: 10.1046/j.1460-9568.2002.02174.x. [DOI] [PubMed] [Google Scholar]
- Ferry B, Di Scala G. Basolateral amygdala NMDA receptors are selectively involved in the acquisition of taste-potentiated odor aversion in the rat. Behav Neurosci. 2000;114:1005–1010. [PubMed] [Google Scholar]
- Gamzu E. The multifaceted nature of taste-aversion-inducing agents: is there a single common feature. In: Barker LM, Best MR, Domjon M, editors. Learning mechanisms in food selection. Houston: Baylor University Press; 1977. pp. 477–502. [Google Scholar]
- Golden GJ, Houpt TA. D-cycloserine potentiates acquisition, but not reversal, of conditioned flavor-taste preference learning. Appetite. 2005;44:351. [Google Scholar]
- Guthmann A, Herbert H. Expression of N-methyl-D-aspartate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei and in selected pontomedullary brainstem nuclei. J Comp Neurol. 1999;415:501–517. [PubMed] [Google Scholar]
- Gutierrez H, Hernandez-Echeagaray E, Ramirez-Amaya V, Bermudez-Rattoni F. Blockade of N-methyl-D-aspartate receptors in the insular cortex disrupts taste aversion and spatial memory formation. Neurosci. 1999;89:751–758. doi: 10.1016/s0306-4522(98)00360-1. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Gallagher M. Taste-potentiated odor conditioning: impairment produced by infusion of an N-methyl-D-aspartate antagonist into basolateral amygdala. Behav Neurosci. 1995;109:663–668. doi: 10.1037//0735-7044.109.4.663. [DOI] [PubMed] [Google Scholar]
- Henderson G, Johnson JW, Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-D-aspartate receptor. J Physiol. 1990;430:189–212. doi: 10.1113/jphysiol.1990.sp018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Wessel TC, Joh TH, Smith GP. Increased c-Fos expression in the rat nucleus of the solitary tract after conditioned taste aversion formation. Neurosci Lett. 1994;172:1–5. doi: 10.1016/0304-3940(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Koehnle TJ, Rinaman L. Progressive postnatal increases in Fos immunoreactivity in the forebrain and brain stem of rats after viscerosensory stimulation with lithium chloride. Am J Physiol. 2007;292:R1212–1223. doi: 10.1152/ajpregu.00666.2006. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Biala G. Effects of the NMDA/glycine receptor antagonist, L-701,324, on morphine- and cocaine-induced place preference. Pol J Pharmacol. 1999;51:323–330. [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Differential modulation of brain immediate early genes by intraperitoneal LiCl. Neuroreport. 1995;7:289–293. [PubMed] [Google Scholar]
- Land C, Riccio DC. d-Cycloserine: effects on long-term retention of a conditioned response and on memory for contextual attributes. Neurobiol Learn Mem. 1999;72:158–168. doi: 10.1006/nlme.1998.3897. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Aigner TG. The glycine/NMDA receptor antagonist HA-966 impairs visual recognition memory in rhesus monkeys. Brain Res. 1996;731:72–78. doi: 10.1016/0006-8993(96)00463-5. [DOI] [PubMed] [Google Scholar]
- Meachum CL, Bernstein IL. Behavioral conditioned responses to contextual and odor stimuli paired with LiCl administration. Physiol Behav. 1992;52:895–899. doi: 10.1016/0031-9384(92)90368-c. [DOI] [PubMed] [Google Scholar]
- Meachum CL, Bernstein IL. Conditioned responses to a taste conditioned stimulus paired with lithium chloride administration. Behav Neurosci. 1990;104:711–715. doi: 10.1037//0735-7044.104.5.711. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. CNQX but not NBQX prevents expression of amphetamine-induced place preference conditioning: a role for the glycine site of the NMDA receptor, but not AMPA receptors. J Pharmacol Exp Ther. 1999;290:9–15. [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moraes Ferreira VM, Morato GS. D-cycloserine blocks the effects of ethanol and HA-966 in rats tested in the elevated plus-maz. Alcohol Clin Exp Res. 1997;21:1638–1642. [PubMed] [Google Scholar]
- Myhrer T. Evidence for activation of NMDA receptors when memory function is reinstated in rats with glutamatergic temporal systems disrupted. Brain Res. 1994;662:263–267. doi: 10.1016/0006-8993(94)90823-0. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav. 1973;10:73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- Navarro N, Cubero I. Lateral parabrachial lesions impair lithium chloride-induced aversive responses but not saccharin-induced flavor preference. Brain Res. 2003;990:195–202. doi: 10.1016/s0006-8993(03)03530-3. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Watanabe S. Intrahippocampal administration of a glycine site antagonist impairs working memory performance of rats. Eur J Pharmacol. 1994;253:183–187. doi: 10.1016/0014-2999(94)90776-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci. 1997;17:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Houpt TA. Dynamics of c-Fos and ICER mRNA expression in rat forebrain following lithium chloride injection. Molec Brain Res. 2001;93:113–126. doi: 10.1016/s0169-328x(01)00173-5. [DOI] [PubMed] [Google Scholar]
- Stafstrom-Davis CA, Ouimet CC, Feng J, Allen PB, Greengard P, Houpt TA. Impaired conditioned taste aversion learning in spinophilin knockout mice. Learn Mem. 2001;8:272–278. doi: 10.1101/lm.42101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele RJ, Stewart MG. 7-Chlorokynurenate, an antagonist of the glycine binding site on the NMDA receptor, inhibits memory formation in day-old chicks (Gallus domesticus) Behav Neural Biol. 1993;60:89–92. doi: 10.1016/0163-1047(93)90145-8. [DOI] [PubMed] [Google Scholar]
- Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- Temple MD, Hamm RJ. Chronic, post-injury administration of D-cycloserine, an NMDA partial agonist, enhances cognitive performance following experimental brain injury. Brain Res. 1996;741:246–251. doi: 10.1016/s0006-8993(96)00940-7. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- Tucci S, Rada P, Hernandez L. Role of glutamate in the amygdala and lateral hypothalamus in conditioned taste aversion. Brain Res. 1998;813:44–49. doi: 10.1016/s0006-8993(98)00884-1. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration of intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, H T, Saito H, Abe K. Involvement of glycine site associated with the NMDA receptor in hippocampal long-term potentiation and acquisition of spatial memory in rats. Brain Res. 1992;582:58–64. doi: 10.1016/0006-8993(92)90316-2. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. c-Fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport. 1992;3:1049–1052. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Morimoto T, Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Res. 2000;869:15–24. doi: 10.1016/s0006-8993(00)02397-0. [DOI] [PubMed] [Google Scholar]


