Abstract
We have previously described an analog peptide of type II collagen (CII) that can suppress collagen-induced arthritis (CIA). This analog peptide represents CII245-270, the immunodominant epitope of CII, but with substitutions at 260, 261, and 263 - CII245-270 (A260, B261, and N263) (A9). To elucidate the mechanisms responsible for suppression, we used mice transgenic for a collagen-specific T cell receptor (TCR). When we found that APCs pulsed with A9 failed to induce T cell phosphorylation of TCR-? and ZAP-70, we explored alternative signaling pathways. We determined that A9 instead induced phosphorylation of spleen tyrosine kinase (Syk). The importance of Syk was confirmed by the use of chemical Syk inhibitors, which blocked both cytokine secretion and activation of GATA-3 mediated by peptide A9. In summary, T cells use an alternative pathway in response to A9 that involves Syk. This novel T cell pathway may represent an important means for altering T cell phenotypes.
Keywords: Collagen II, T cells, altered peptide ligands, T cell signaling, Syk (spleen tyrosine kinase), autoimmunity
Introduction
We have used the animal model of collagen-induced arthritis (CIA) to understand the pathogenic mechanisms by which autoimmunity to CII leads to inflammatory arthritis. Our goal is to develop a specific immunotherapy that is capable of regulating the autoimmune response and suppress arthritis. In mice, CIA is strongly linked to the class II MHC genes and is restricted primarily to mice with an H-2q or H-2r haplotype [1; 2]. This restriction is related to the ability of these immune response genes to bind to a limited number of immunogenic epitopes on CII and to the MHC influence on the development of the T cell repertoire [2]. For H-2q the dominant epitope is located within CII245-270 and the core determinant is CII260-267. Within this core, the amino acids at positions 260 and 263 act as anchors to bind the epitope to the MHC. Other amino acids within the core epitope interact with the T cell receptor (TCR) to induce T cell activation via the MHC/Peptide/TCR trimolecular complex.
We have previously determined that an analog peptide representing CII245-270, but containing amino acid substitutions at positions 260, 261, and 263, CII245-270 (A260, B261, and N263) (A9), could profoundly suppress CIA [3; 4]. The mechanism by which A9 exerts its suppressive effect is not fully understood, but is likely related to T cell activation induced by A9, since the suppressive effect could be transferred with A9 activated T cells. We have shown that culture of T cells isolated from CII immunized DBA/1 mice with A9 caused a shift in the cytokine response towards a Th2-type profile [3]. It appears A9 is capable of redirecting T cells to function as arthritis suppressing cells.
In order to study T cell responses to collagen in more detail, we developed a TCR transgenic mouse (qCII24) which expresses a TCR specific for the immuno-dominant CII epitope. The majority of circulating T cells in qCII24 mice express the CII reactive TCR. These mice are highly susceptible to CIA and develop severe arthritis beginning 10 days after immunization with CII and achieving an incidence of 100% [5]. Although qCII24 mice develop a very severe form of CIA, we found that it is still possible to suppress disease by treating them with A9. Using T cells isolated from qCII24 mice, we compared early T cell receptor signaling pathways in response to CII245-270 (A2) with those induced by A9. We found that A2 induced substantial phosphorylation of the TCRζ chain and ZAP-70 while stimulation with A9 was unable to induce significant phosphorylation of either one. This suggested that TCR signaling must be occurring through an alternative pathway. Further investigations showed that pathway to involve spleen tyrosine kinase (Syk) which is not usually associated with T cell signaling.
Experimental Procedures
Mice
DBA/1 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice transgenic for a CII-specific TCR-Va11.1/Vß8.3 were established and bred in the animal core facility of the Rheumatic Diseases Research Core Center, University of Tennessee as described previously [2]. All mice were maintained in groups of six in polycarbonate cages and fed standard rodent chow (Ralston Purina Co., St. Louis, Mo.) and water ad libitum. The environment was specific pathogen-free and sentinel mice were tested routinely for mouse hepatitis and Sendai viruses.
CD4+ T cell isolation and activation
Spleens were collected from Vα11.1/Vβ8.3Tg mice and the single-cell suspension was prepared by mechanical disruption in complete DMEM medium (DMEM supplemented with 10 % FCS, 100 IU/ml of penicillin, 100 μg/ml streptomycin, 2.5 μM ß-mercaptoethanol and 2 mM L-glutamine). CD4+ naive T cells were labeled by immunomagnetic beads using a CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, Ca) followed by negative selection over a autoMACS isolation system (Miltenyi). The purity of the recovered CD4+ T cells was determined by flow cytometry after staining with anti-CD4+ mAb and were >95% pure as determined by flow cytometry. Cells were cultured for varying lengths of time (30 seconds to 60 minutes) with APC (I-Aq-positive splenocytes) which have been prepulsed with A2, A9, or other analog peptide(s). In some experiments, after a short culture period at 37 °C, the cells were collected, lysed in lysis buffer, and insoluble materials were removed by centrifugation at 10,000 x g at 4 °C for 15 minutes.
Cytokine Assays
To test for patterns of cytokine response, T cells were cultured with various peptides in the presence of APCs. Supernatants were collected 72 hours later and analyzed for quantities of Th1 (IFN-?, IL-2), Th17 (IL-17) and Th2 (IL-4, IL-5, IL-10, and TGF-ß) cytokines using a multiplexed ELISA (Bioplex, BioRad).
Analysis of protein phosphorylation
Whole cell lysates were separated on a SDS-PAGE and electrotransferred onto nitrocellulose membranes. After transfer, the membrane was blocked in TBS (tris buffered saline) containing 3% No-fat dry milk for 2 hours, incubated with phospho specific antibodies in TBS-Tween 20 /3% milk overnight. The membrane was then incubated with a secondary antibody (Amersham) for 1 hour and subjected to Enhanced Chemiluminescence detection (ECL Western Blot kit, Amersham) according to the manufacturer’s protocol. For detection of protein levels, the membranes were re-stripped and blocked with 3% no-fat milk, incubated with anti-pan antibodies followed by ECL detection.
Flow cytometric detection of intracellular phospho-proteins in qCII24 T cells was carried out as follows. The magnetic purified CD4+ T cells were stimulated with peptide-prepulsed antigen presenting cells at different time periods (1 to 60 minutes). Cells were fixed with 1% formaldehyde and permeabilized with methanol. The fluorescent-conjugated phospho-specific antibodies as well as antibodies to T cell surface markers (CD3, CD4 and TCR-ß) were added to the cell preparations and incubated at room temperature for 1 hour. The intracellular phosphorylation-state was analyzed on a FacsCalibur flow cytometer (Becton Dickinson) using CellQuest and FlowJo software.
Reagents
The peptide representing the immunodominant determinant of CII245-270 (ATGPLGPKGQTGEBGIAGFKGEQGPK), is designated peptide A2 [6]. B stands for 4-hydroxyproline. A2 and its analog peptide A9, containing three specific amino acid substitutions at positions 260, 261, and 263, CII245-270 (A260, B261, and N263), were chemically synthesized by a solid-phase procedure and purified by high performance liquid chromatography [7]. The two chemical Syk inhibitors used were piceatannol (Sigma) and sulfonamide-3 (designated Syk I) or 3-(1-Methyl-1H-indol-3-yl-methylene)-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide) (Calbiochem). Antibodies and anti-phospho specific antibodies against Erk, P38, JNK/SAPK, Zap 70 and Syk were obtained from Cell Signaling Technology, Inc. (Beverly, MA) and the anti-GATA-3 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, Ca.).
Statistics
Statistical analysis was performed using the Student’s T test.
Results
Cytokine Response to A9
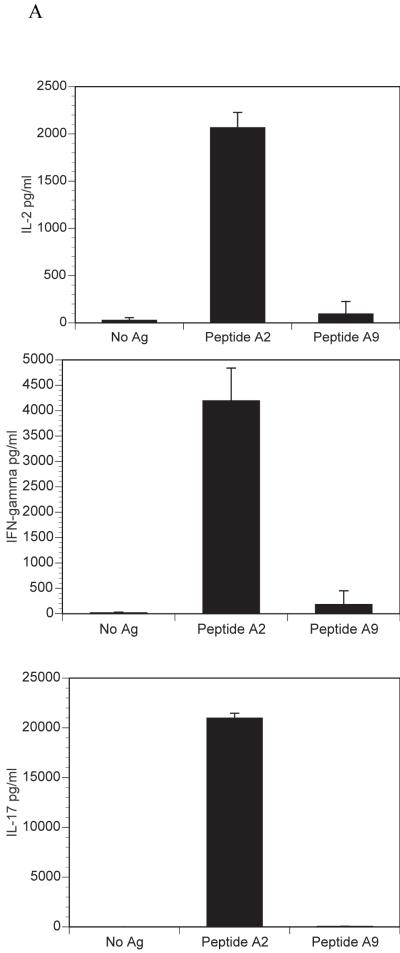
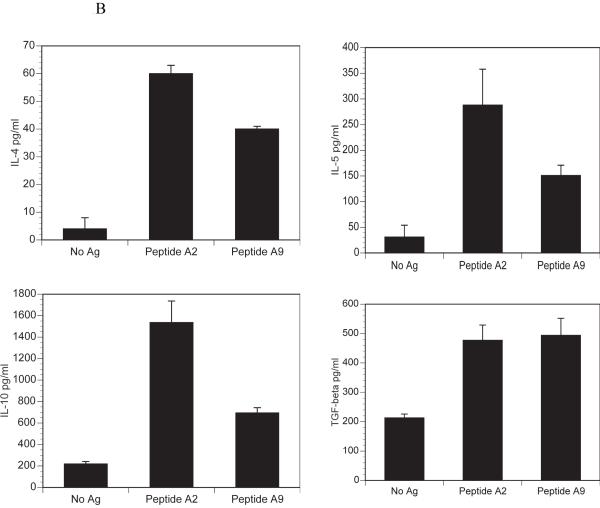
We have previously described the development of a mouse, qCII24, which expresses a transgenic TCR that is specific for the immunodominant CII epitope, CII245-270, (A2). These mice provide an excellent source of CII-reactive T cells for signaling studies. In order to test for cytokine responses to collagen peptides, CD4+ T cells were isolated from the spleens of naïve mice and cultured with either A2 or A9 in the presence of antigen presenting cells. Culture with A2 induced significant amounts of the proinflammatory cytokines IL-2, IL-17 and IFN-?, while A9 did not. On the other hand, both peptides induced the Th2 cytokines, IL-4, IL-5, IL-10, and TGF-ß. (Figure 1). These patterns of cytokines responses are similar to those previously reported in DBA/1 mice following immunization with CII and treatment with A9 [3]. Together, the data confirm that CD4+ T cells from qCII24 transgenic mice respond to the A9 peptide with a predominantly Th2 phenotype, although the levels of IL-4, IL-5 and IL-10 are less than those induced by wild type peptide. The ability to induce the secretion of Th2 cytokines may explain the profound suppressive effects A9 has on the development of CIA.
Figure 1. Cytokine responses to A2 and A9.
Spleen cells were isolated from qCII24 transgenic mice and cultured with or without 100 μg/ml of each peptide for 6 days. Supernatants were collected and tested for the presence of each cytokine using a multiplexed ELISA. Results shown represent the means of three separate experiments and are expressed in pg/ml of supernatant fluid. Panel A represents the response to inflammatory cytokines (IL-2, IFN-?, and IL-17), while Panel B represents the response to suppressive cytokines (IL-4, IL-5, IL-10, and TGF-ß). The response to A2 but not to A9 was significantly greater than background for each cytokine tested in panel A, p = 0.001. The cytokines in panel B gave a significant response above background to both A2 and A9, p= 0.05. Although only one dose of peptide is shown, we have tested multiple doses which have demonstrated similar patterns. Moreover, concentrations of A2 as low as 1μg/ml still elicited greater Th1 and Th17 cytokine responses than Th2 cytokines (103 pg/ml of IFN-?; 1636 pg/ml of IL-17; 3 pg/ml of IL-4; 47 pg/ml of IL-10) confirming that the cytokine pattern elicited by A9 could not be duplicated by simply lowering the dose of A2. The cytokine response to A9 is I-Aq restricted, as CII-reactive T cells from I-Ar mice do not respond to A9 (data not shown).
Early T cell signaling with A2 and A9
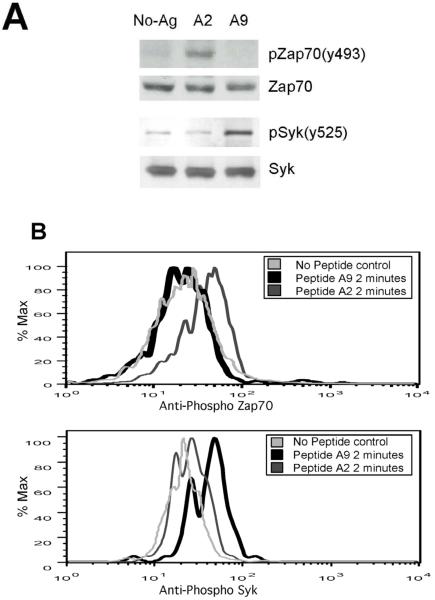
In order to understand the molecular basis for the difference in the T cell response to A2 contrasted with A9, we explored the intracellular T-cell signaling pathways initiated by both peptides. CII-reactive T cells cultured with APCs previously pulsed with A2 induced strong phosphorylation of the 70kD band of ZAP-70 while A9 failed to do so (Figure 2A). Since A9 clearly induces IL-4, IL-5, IL-10 and TGF-ß, it must act through an alternative pathway. Further analysis revealed culture of T cells with A9 pulsed APCs caused activation of spleen tyrosine kinase (Syk), a kinase ordinarily utilized by mast cells and B cells but recently reported to play a critical role in T cells from patients with autoimmune diseases [8](Figure 2A). In order to confirm that Syk was activated in CD4+ cells, we used flow cytometry. In these experiments APCs were prepulsed with either A2 or A9, T cells added and the cells fixed, permeabilized and labeled with antibodies specific for phospho-ZAP-70 and phospho-Syk. By gating on CD-4 cells we were able to confirm that the T cells stimulated with A2 responded by phosphorylation of ZAP-70 while stimulation with A9 resulted in phosphorylation of Syk (Figure 2B).
Figure 2. Activation of ZAP-70 and Syk by A2 and A9.
A) The qCII24 CD4+ T cells were activated by APCs pre-pulsed with 100 μg/ml of A2, A9 or no peptide (No Ag) for 5 minutes. This time point was selected based on a time course analysis showing that the peak activation occurred between two to five minutes after stimulation. Whole cell lysates were collected and the proteins were separated by SDS-PAGE. The proteins weretransferred onto PVDF membranes and analyzed first using antibodies specific for ZAP-70 (pZAP-70-y493) and Syk (pSyk-y525). The membrane was then stripped and reprobed with antibodies to total ZAP-70 (ZAP-70) and total Syk (Syk) respectively. B). The T cells were stimulated by APCs pre-pulsed with 100 μg/ml of A2, A9 or no peptide (Control) for 2 minutes. The cells were then fixed, permeabilized, stained with an antibody specific for phospho-ZAP-70 (top panel) or an antibody specific for phospho-Syk (bottom panel) and analyzed by flow cytometry. The histograms were gated on CD4+ T cells.
Testing for phospho-specific MAP kinase components
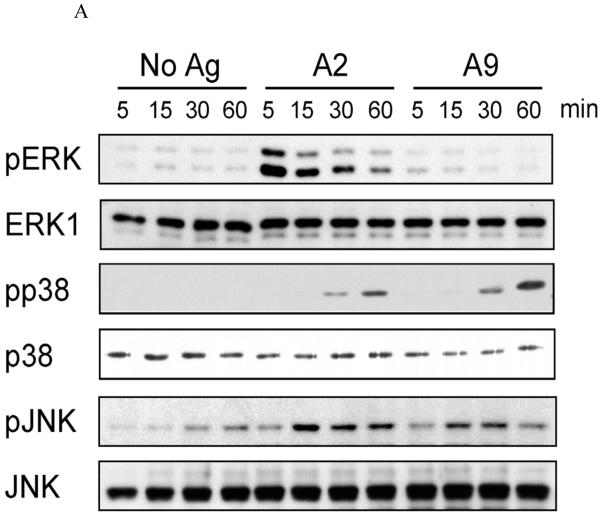
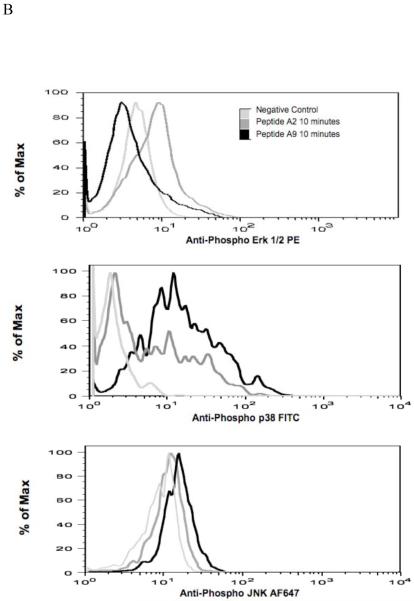
Mitogen-activated protein kinases (MAPKs) are thought to play a key role in the transmission of signals from the outside of the T cell to AP-1 binding DNA sequences inside the nucleus. Therefore, we investigated the T cell activation of each of the three major components of MAPK, (ERK, p38 and JNK). The T cells exposed to A2 had rapid phosphorylation of ERK1/2 while exposure to A9 had no effect (Figure 3A). On the other hand, both p38 and JNK were phosphorylated following exposure to either A2 or A9. Flow cytometric analysis confirmed the results obtained by Western blot analysis, showing that CD4+ T cells were activated in response to either A2 or A9 stimulations (Figure 3B). These data clearly showed that MAP kinase activation pattern by A9 was quite different than that induced following exposure to WT A2. We did detect some minor variations betweenthe phospho flow data and the western blots. For example, phospho flow analysis detected p38 activity at an earlier time point than the western blot. This may represent a greater sensitivity of this technique. Similarly, we noted that although both A2 and A9 clearly activated pJNK, the activation by A9 was less than that of WT A2 peptide by western blot while the opposite was documented by phospho flow. Despite these minor differences, these data clearly showed that exposure to A2 induced a very different MAP kinase activation pattern than that induced following exposure to A9 peptide.
Figure 3. Differential activation of MAPKs by A2 and A9.
A). CD4+ T cells from the spleens of qCII24 transgenic mice were incubated with APCs pre-pulsed with 100 μg/ml of A2, A9, or no peptide (No Ag) for the time periods indicated. Western blot analysis of the total cell lyates was performed using specific antibodies against phospho-Erk(pErk)/total Erk-1/2 (Erk), phosph-P38 (pP38)/total P38 (P38) and phospho-JNKp52/total JNKp52 (Figure 4A). B). CD4+ T cells from qCII24 mice were incubated with APCs pre-pulsed with 100 μg/ml of A2 or A9 at 37 °C for ten minutes. The cells were fixed and permeabilized before staining with an antibody specific for phospho Erk 1/2, phospho-p38, or phospho-JNK. The histograms were gated on CD4+ cells. Although the flow data shown are representative diagrams, each experiment was performed three times and the mean fluoresence values are indicated as follows: ERK 1/2 - - no antigen 12.4 ± 5, A2 19.8 ± 9, and A9 9.9 ± 7. p38 -- no antigen 17.3 ± 5, A2 41.5 ± 8, and A9 49 ± 12; JNK no antigen 14.5 ± 5, A2 19.5 ± 8, A9 22.6 ± 7. The activation of ERK1/2 was significantly greater than background when activated by A2, p = 0.05 but not by A9. The p38 activation was significantly greater than background when activated by both A2 and A9, p = 0.01.
Syk inhibitors block A9 but not A2, induced Signaling
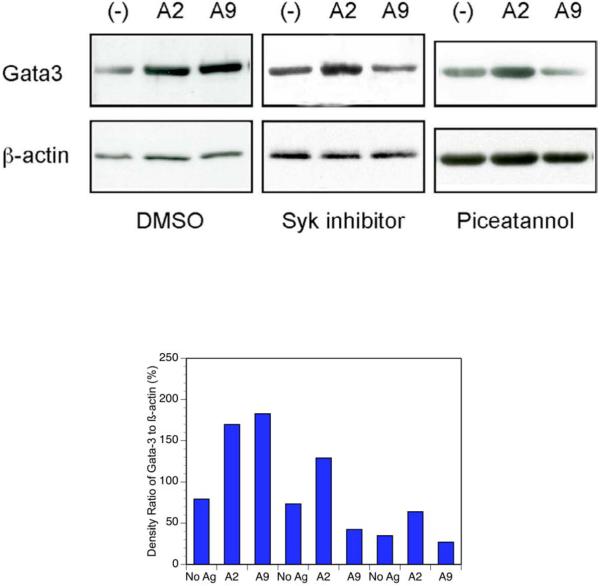
Based on our findings, we hypothesized that A9 induces an alternative signaling pathway by phosphorylating Syk, thus bypassing theTCR-?/Zap70 pathway that is induced by A2. To test this, we used both piceatannol and sulfonamide-3 (Syk I) to block Syk and then examined the activation of the zinc finger transcription factor GATA-3, which is known to upregulate the promoters of Th2 cytokines in T cells. CD4+ transgenic T cells were treated with each inhibitor in the presence of antigen presenting cells pre-pulsed with A2 or A9. After two hours the cells were lysed and the lysates probed for the presence of GATA-3. Stimulation of the T cells with A9 resulted in activation of GATA-3. However, in the presence of the Syk inhibitors this activation was blocked (Figure 4). As expected, activation of the T cells with A2 resulted in strong activtion of GATA-3, and this effect was not blocked by piceatannol or Syk I.
Figure 4. Inhibition of GATA-3 by Syk inhibitors.
CD4+ T cells from qCII24 transgenic mice were cultured with 100 μg/ml of either A2 or A9 peptide, or without any peptide (-) for 6 hours in the presence of a Syk inhibitor (Syk I) (10 pM), Piceatannol (300 μM), or DMSO as control. After culture, the cells were collected and the extracts were subjected to immunoblot analysis using an antibody against GATA-3 (top panel). The same membranes were then stripped and re-blotted with anti-β-actin antibody (lower panel). The results shown are representative of data obtained from three separate experiments. Densitometry of the results is displayed below the gel.
These results confirmed that A9 causes T cell activation through an “alternative” Syk signaling pathway, which results in activation of a different set of MAPKs as well as GATA-3. Conversely, A2 causes activation of T cells through a TCRζ/ZAP-70 signaling pathway which is the classical TCR signal cascade.
Suppression of cytokines with Syk inhibitors
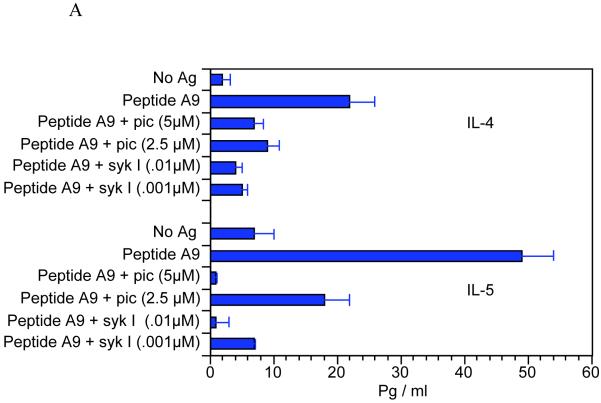
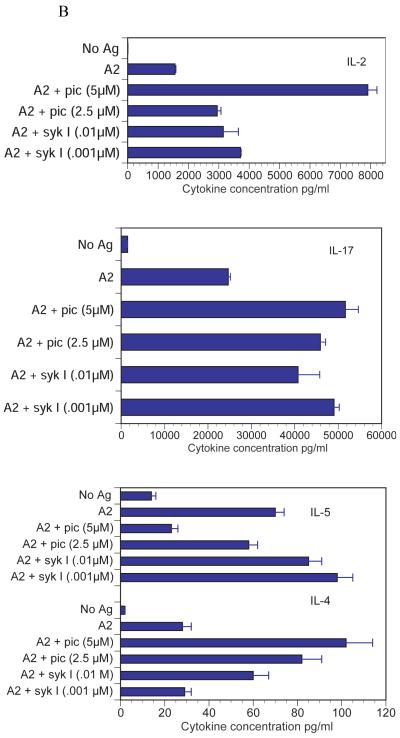
Confirmation that the differences in cell signaling through Syk were responsible for the change in T cell function required demonstration that the cytokine responses correlated with activation of Syk. For this pupose we used two Syk inhibitors, sulfonamide-3 (Syk I) and piceatannol. APCs were prepulsed with CII or A9 and qCII24 T cells added along with the inhibitors. Supernatants were collected and tested for the presence of cytokines using a multiplexed ELISA. We found that the addition of the Syk inhibitors greatly reduced the IL-4 and IL-5 cytokine responses to A9, while having little effect on the IL-4 and IL-5 responses to A2 (Figure 5). The only exception was the inhibition of the IL-5 response to A2 in the presence of the highest dose of piceatannol. Since this effect was only seen with one of the two inhibitors, its significance is unclear. Similarly, the two Syk inhibitors did not decrease the inflammatory cytokine responses of A2. In some concentrations, the Syk inhibitors actually enhanced the inflammatory cytokine responses following exposure to A2. It is possible that Syk plays an inhibitory role in T cell responses to the wild type peptide. On the other hand, it is equally plausible that disruption of Syk leads to disordered downstream effects that distort typical T cell signaling pathways. In summary, these data clearly confirm that Syk is essential for T cells to secrete Th2 cytokines in response to the analog peptide A9.
Figure 5. Effects of Syk inhibitors on cytokine responses to A2 and A9.
Splenocytes from qCII24 transgenic mice were cultured with antigen presenting cells prepulsed with 100 μg/ml of either A9 peptide (Figure 5A) or A2 peptide (Figure 5B) for 6 days in the presence or absence of the indicated concentrations of piceatannol (Pic), or sulfonamide-3, (Syk I). Supernatants were collected and tested for the presence of each cytokine using a multiplexed ELISA. Results are expressed in pg/ml of supernatant fluid. (Only the Th2 cytokine responses are shown for A9, because this peptide was unable to induce any significant inflammatory cytokine responses. Neither did the presence of the syk inhibitors induce their secretion.) The Syk inhibitors both significantly inhibited the IL-4 response to A9, p = 0.01 and the IL-5 response to A9 p = 0.005. Neither inhibitor was able to suppress the cytokine responses to A2, with the exception of only the highest dose of piceatannol, p = 0.05, against IL-5. IL-2 is shown as a representative Th1-type cytokine. However, the results for the syk inhibitor were similar for the other Th1 cytokines (for example the IFN-? response to A2 was 42,000 ± 2000 pg/ml; while in the presence of pic 5μM the results were 51,00 0 ± 3,000 pg/ml and in the presence of sykI 0.01 μM the results were 47,000 ± 2500 pg/ml).
Discussion
We report that a collagen-reactive TCR uses different signaling pathways in reaction to a collagen analog and wild type peptide despite presentation by the same I-Aq. If presented with antigen presenting cells pulsed with A2, qCII24 T cells secrete a full spectrum of cytokines, and develop fully phosphorylated TCR zeta and ZAP-70. When exposed to A9, Syk becomes phosphorylated and the T cells become polarized to secrete Th2-type cytokines. Analysis of down-stream MAP Kinase phosphorylation reveals even greater differences. While A2 causes a rapid phosphorylation of ERK, together with phosphorylation of p38 and JNK, T cells stimulated with A9 develop phosphorylation of JNK and p38 without detectable phosphorylation of ERK. These signaling differences help explain the molecular basis for the paradox that polarization of T cells with A9 causes a vigorous Th2 cytokine response without stimulating the classical T cell signaling pathway. Similarly, Itoh and coworkers report that polarized Th2 cells had little or no detectable Zap-70 phosphorylation despite detectable cytokine responses [9]. These findings imply a quantitative change in the molecular properties of the T cells in response to antigens which cause polarization to a primarily Th2 response. Although the T cell signaling pathways have been studied extensively using T cell lines or clones, our studies are unique in our use of natural (TCR-Tg) CD4+ T cells tested directly ex vivo in response to specific peptides
Current models of antigen/MHC induced T-cell activation suggest that there is a sequential interaction of Src and ZAP-70/Syk protein tyrosine kinases (PTKs) with the TCR/CD3/complex. TCR engagement causes activation of the Src family PTKs Lck/Fyn, which phosphorylate the tyrosines present in the immunoreceptor tyrosine activation motif (ITAM) [10]. The ZAP-70/Syk PTKs then bind to the phosphorylated ITAMs via their respective SH2 domains and activate downstream signaling cascades. ZAP-70 and Syk are structurally homologous; and are composed of 2 tandem arranged SH2 domains and share more than 50% sequence identity. These 2 PTKs have overlapping functions but they have distinct expression profiles. ZAP-70 is expressed exclusively in thymocytes, T cells, and natural killer (NK) cells, whereas Syk is expressed in a wide variety of hematopoietic cells including B cells and mast cells as well as peripheral T cells [11; 12; 13]. Although Syk is 100 fold more potent as a kinase than ZAP-70, ZAP-70 is a much more efficient phosphorylator of the TCR? chain.
It has been shown that Syk is expressed at high levels in some human CD4+ effector T cells [8; 14; 15]. Although its importance in B cell and mast cell signaling has been extensively documented, its role in T cell function is poorly understood. Lupus patients, for example, have strikingly reduced expression of CD3-? in effector CD4+ T cells [8; 16; 17]. Moreover, certain patients with SLE preferentially phosphorylate Syk rather than ZAP-70 [14; 17; 18]. Investigators have previously hypothesized involvement of an alternative signaling pathway in T cell activation and have implicated various molecules, including members of the Src family and of the Syk/ ZAP-70 family [19; 20; 21]. It has also been shown that Syk may be involved in signaling through the IL-2 receptor and its activation may prevent T cell apoptosis [22]. However, the functional importance of Syk and its link to Th2 cytokine production has not been previously recognized.
Although the precise mechanism by which A9 peptide exerts its effect is not clear, our data and that of other investigators have indicated that minor variations in the peptide binding affinity or in the physicochemical properties of amino acid residues involved in MHC binding and interaction with the TCR can lead to disparate immunological responses [23; 24; 25; 26; 27]. We have determined that two of the amino acids that give A9 its unique properties are involved in MHC (I-Aq) binding, CII260 extends into the binding pocket for p1 and CII263 extends into the pocket at p4 as confirmed by binding studies showing that A9, which contains substitutions at 260 and 263, binds less strongly to I-Aq than wild type CII256-276 analog peptides. Of the amino acids altered in A9, only CII261 is positioned to interact with the TCR. The changes in MHC binding differentiate A9 from previously described APL that have altered amino acids at peptide positions that are involved only in TCR interaction.
Reduced binding is likely to have several consequences: 1) very low density of MHC/A9 on the presenting cell surface and 2) possible alteration in TCR interaction. Although it has previously been thought that MHC binding was mostly independent of MHC/Peptide surface conformation, new technology using MHC/peptide tetramers reveal that changes in the residues interacting with the P1 and P4 MHC binding pockets can induce subtle but important stereochemical changes on the neighboring residues positioned to interact with the TCR [28; 29].
An emerging hypothesis is that the effect of new biologic therapies, such as peptides or antibodies, are linked to their ability to quantitatively and qualitatively modulate the clustering of target membrane receptors and signaling kinases within the plasma membrane. This activity would be at the level of the so-called “immunologic synapse.” In this model, a reduced avidity of interaction with either the MHC or the TCR might cause the antigen receptor within the immunologic synapse to cluster with Syk/FceRI?, rather than ZAP-70/?. Alternatively an analog peptide may be incapable of inducing an immunologic synapse to form. These differences may explain the two fundamentally different outcomes that occur following culture of CII-specific T cells with either WT A2 (induction of the full spectrum of cytokines) or suppressive A9 (induction of only the suppressive cytokines). We cannot exclude a third possibility, that A9 activates another receptor on the surface of T cells which preferentially utilizes Syk rather than Zap-70.
A shift to a Th2 cytokine profile is significant because Th2 cytokines have inhibitory effects on CIA [30; 31; 32; 33; 34; 35]. IL-10 is effective in down-regulating arthritis when administered to mice [36], yet it does not duplicate the effects of IL-4. Our previous data is consistent with the concept that IL-4 has a unique role in the suppression of arthritis that is only partially duplicated by other Th2 cytokines in the absence of IL-4 [37]. Such observations support out hypothesis that antigen interaction with the TCR in the context of the MHC can influence the cytokine profiles which are eventually produced by T cells.
Although the use of APL in the treatment of human disease is limited [38; 39; 40], recent evidence suggests that they can induce regulatory T cells that play a role in modulating autoimmune arthritis [41; 42]. Prakken and coworkers described treating arthritis patients with a dnaJP1 peptide orally for 6 months. Immunological analysis showed an intriguing change from proinflammatory to regulatory T cell function with increased production of IL-4 and IL-10 [42].
In summary, we more precisely define the mechanism of action of an APL which modulates arthritis in the collagen induced arthritis animal model. An APL based on a CII peptide uses a unique T cell signaling pathway to down-regulate arthritis, inducing phosphorylation of Syk rather than ZAP-70, and signaling through a pathway not previously implicated in the regulation of arthritis. The functional importance of Syk was confirmed using chemical inhibitors of Syk. Activation of this alternative pathway is a novel observation and may represent an important means by which the phenotype of the responding T cell is altered. A full understanding of the mechanism by which A9 prevents arthritis may lead to development of innovative immunotherapeutic approaches to antigen specific treatment of autoimmunity. APLs represent a safer therapy because the mechanism of action targets the cartilage of the joint without aggravating existing disease. Our hope is that the information gained from this work will allow us to understand the mechanism of an APL applicable for use in rheumatoid arthritis. We believe the new treatment modality will be safer and more precise by triggering a select population of inhibitory T cells.
Acknowledgments
This work was supported, in part, by USPHS Grants AR-39166, AR-43589, and program-directed funds from the Department of Veterans Affairs and the Arthritis Foundation.
Abbreviations
- CII
Type II collagen
- RA
rheumatoid arthritis
- MHC
major histocompatibility complex
- APC
antigen presenting cell
- CIA
collagen induced arthritis
- APL
altered peptide ligand
- Peptide A2
CII 245-270 (ATGPLGPKGQTGEBGIAGFKGEQGPK)
- Peptide A9
(ATGPLGPKGQTGEBGABGFNGEQGPK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wooley PH, Dillon AM, Luthra HS, Stuart JM, David CS. Genetic control of type II collagen-induced arthritis in mice. Factors influencing disease susceptibility and evidence for multiple MHC-associated gene control. Trans Proc. 1983;15:180–186. [Google Scholar]
- [2].Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–78. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- [3].Myers LK, Tang B, Rosloniec EF, Stuart JM, Chiang TM, Kang AH. Characterization of a peptide analog of a determinant of type II collagen that suppresses collagen-induced arthritis. J Immunol. 1998;161:3589–95. [PubMed] [Google Scholar]
- [4].Myers L, Rosloniec E, Seyer J, Stuart J, Kang A. A synthetic peptide analogue of a determinant of type II collagen prevents the onset of collagen-induced arthritis. J Immunol. 1993;150:4652–4658. [PubMed] [Google Scholar]
- [5].Brand DD, Myers LK, Whittington KB, Latham KA, Stuart JM, Kang AH, Rosloniec EF. Detection of early changes in autoimmune T cell phenotype and function following intravenous administration of type II collagen in a TCR-transgenic model. J Immunol. 2002;168:490–8. doi: 10.4049/jimmunol.168.1.490. [DOI] [PubMed] [Google Scholar]
- [6].Baldwin CT, Reginato AM, Smith C, Jimenez SA, Prockop DJ. Structure of cDNA clones coding for human type II procollagen: The α1(II) chain is more similar to the α1(I) chain than two other α chains of fibrillar collagens. Biochem. J. 1989;262:521–528. doi: 10.1042/bj2620521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosloniec EF, Kang AH, Myers LK, Cremer MA. Collagen-induced arthritis. In: Coico R, Shevach E, editors. Current Protocols in Immunology. Wiley & Sons; New York, NY: 1997. pp. 15.5.1–24. [Google Scholar]
- [8].Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. J Immunol. 2003;171:3325–31. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- [9].Itoh Y, Wang Z, Ishida H, Eichelberg K, Fujimoto N, Makino J, Ogasawara K, Germain RN. Decreased CD4 expression by polarized T helper 2 cells contributes to suboptimal TCR-induced phosphorylation and reduced Ca2+ signaling. Eur J Immunol. 2005;35:3187–95. doi: 10.1002/eji.200526064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183:1053–62. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Simon M, Vanes L, Geahlen RL, Tybulewicz VL. Distinct roles for the linker region tyrosines of Syk in FcepsilonRI signaling in primary mast cells. J Biol Chem. 2005;280:4510–7. doi: 10.1074/jbc.M410326200. [DOI] [PubMed] [Google Scholar]
- [12].Renaudineau Y, Nedellec S, Berthou C, Lydyard PM, Youinou P, Pers JO. Role of B-cell antigen receptor-associated molecules and lipid rafts in CD5-induced apoptosis of B CLL cells. Leukemia. 2005;19:223–9. doi: 10.1038/sj.leu.2403601. [DOI] [PubMed] [Google Scholar]
- [13].Chan AC, van Oers NS, Tran A, Turka L, Law CL, Ryan JC, Clark EA, Weiss A. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J Immunol. 1994;152:4758–66. [PubMed] [Google Scholar]
- [14].Krishnan S, Juang YT, Chowdhury B, Magilavy A, Fisher CU, Nguyen H, Nambiar MP, Kyttaris V, Weinstein A, Bahjat R, Pine P, Rus V, Tsokos GC. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. 2008;181:8145–52. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Crispin JC, Kyttaris V, Juang YT, Tsokos GC. Systemic lupus erythematosus: new molecular targets. Ann Rheum Dis. 2007;66(Suppl 3):iii65–9. doi: 10.1136/ard.2007.078493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, Tsokos GC. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172:7821–31. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- [17].Juang YT, Wang Y, Jiang G, Peng HB, Ergin S, Finnell M, Magilavy A, Kyttaris VC, Tsokos GC. PP2A dephosphorylates Elf-1 and determines the expression of CD3zeta and FcRgamma in human systemic lupus erythematosus T cells. J Immunol. 2008;181:3658–64. doi: 10.4049/jimmunol.181.5.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Crispin JC, Tsokos GC. Transcriptional regulation of IL-2 in health and autoimmunity. Autoimmun Rev. 2009;8:190–5. doi: 10.1016/j.autrev.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Letourneur F, Klausner RD. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- [20].Taylor N, Steinberg M, Oumeya A, Swainson L, Merida P, Noraz N, Di Bartolo V, Pelletier L. T cell receptor-induced phosphorylation of the {zeta} chain is efficiently promoted by ZAP-70 but not Syk. Blood. 2004 doi: 10.1182/blood-2003-12-4314. [DOI] [PubMed] [Google Scholar]
- [21].Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, Geiger TL, Doherty P, Ihle JN. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20:37–46. doi: 10.1016/s1074-7613(03)00351-0. [DOI] [PubMed] [Google Scholar]
- [22].Jiang K, Zhong B, Ritchey C, Gilvary DL, Hong-Geller E, Wei S, Djeu JY. Regulation of Akt-dependent cell survival by Syk and Rac. Blood. 2003;101:236–44. doi: 10.1182/blood-2002-04-1251. [DOI] [PubMed] [Google Scholar]
- [23].Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science (Wash. DC) 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- [24].Evavold BD, Sloan-Lancaster J, Allen PM. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunology Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- [25].Evavold BD, Sloan-Lancaster J, Hsu BL, Allen PM. Separation of T helper 1 clone cytolysis from proliferation and lymphokine production using analog peptides. J. Immunol. 1993;150:3131–3140. [PubMed] [Google Scholar]
- [26].Kersh GJ, Allen PM. Structural basis for T cell recognition of altered peptide ligands: a single T cell receptor can productively recognize a large continuum of related ligands. J. Exp. Med. 1996;184:1259–1268. doi: 10.1084/jem.184.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alexander J, Snoke K, Ruppert J, Sidney J, Wall M, Southwood S, Oseroff C, Arrhenius T, Gaeta FCA, Colón SM, Grey HM, Sette A. Functional consequences of engagement of the T cell receptor by low affinity ligands. J Immunol. 1993;150:1–7. [PubMed] [Google Scholar]
- [28].Nepom GT. Tetramer Analysis of Human Autoreactive CD4-Positive T Cells. Adv Immunol. 2005;88:51–71. doi: 10.1016/S0065-2776(05)88002-2. [DOI] [PubMed] [Google Scholar]
- [29].Mallone R, Kochik SA, Reijonen H, Carson B, Ziegler SF, Kwok WW, Nepom GT. Functional avidity directs T-cell fate in autoreactive CD4+ T cells. Blood. 2005;106:2798–805. doi: 10.1182/blood-2004-12-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Joosten LA, Lubberts E, Helsen MM, Saxne T, Coenen-de Roo CJ, Heinegard D, van den Berg WB. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1:81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim SH, Kim S, Evans CH, Ghivizzani SC, Oligino T, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001;166:3499–505. doi: 10.4049/jimmunol.166.5.3499. [DOI] [PubMed] [Google Scholar]
- [32].Watanabe S, Imagawa T, Boivin GP, Gao G, Wilson JM, Hirsch R. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol Ther. 2000;2:147–52. doi: 10.1006/mthe.2000.0111. [DOI] [PubMed] [Google Scholar]
- [33].Sarvetnick N. Mechanisms of cytokine-mediated localized immunoprotection. J. Exp. Med. 1996;184:1597–1600. doi: 10.1084/jem.184.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shih FF, Mandik-Nayak L, Wipke BT, Allen PM. Massive Thymic Deletion Results in Systemic Autoimmunity through Elimination of CD4+ CD25+ T Regulatory Cells. J Exp Med. 2004;199:323–35. doi: 10.1084/jem.20031137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nishibori T, Tanabe Y, Su L, David M. Impaired Development of CD4+ CD25+ Regulatory T Cells in the Absence of STAT1: Increased Susceptibility to Autoimmune Disease. J Exp Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20. J Immunol. 2001;167:3545–9. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- [37].Myers LK, Tang B, Stuart JM, Kang AH. The role of IL-4 in regulation of murine collagen-induced arthritis. Clin Immunol. 2002;102:185–91. doi: 10.1006/clim.2001.5162. [DOI] [PubMed] [Google Scholar]
- [38].Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- [39].Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L, Rae-Grant A, Castaldo J, Eckert N, Guarnaccia JB, Mills P, Johnson G, Calabresi PA, Pozzilli C, Bastianello S, Giugni E, Witjas T, Cozzone P, Pelletier J, Pohlau D, Przuntek H, Hoffmann V, Bever C, Jr., Katz E, Clanet M, Berry I, Brassat D, Brunet I, Edan G, Duquette P, Radue EW, Schott D, Lienert C, Taksaoui A, Rodegher M, Filippi M, Evans A, Bourgouin P, Zijdenbos A, Salem S, Ling N, Alleva D, Johnson E, Gaur A, Crowe P, Liu XJ. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. 2000;6:1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- [40].Kim HJ, Antel JP, Duquette P, Alleva DG, Conlon PJ, Bar-Or A. Persistence of immune responses to altered and native myelin antigens in patients with multiple sclerosis treated with altered peptide ligand. Clin Immunol. 2002;104:105–14. doi: 10.1006/clim.2002.5258. [DOI] [PubMed] [Google Scholar]
- [41].Leipe J, Skapenko A, Lipsky PE, Schulze-Koops H. Regulatory T cells in rheumatoid arthritis. Arthritis Res Ther. 2005;7:93. doi: 10.1186/ar1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J, Amox D, Roord S, de Kleer I, Bonnin D, Lanza P, Berry C, Massa M, Billetta R, Albani S. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101:4228–33. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]