Abstract
The efficient degradation of internalized particulate matter is a principal objective of the macrophage’s phagosome. Assessment of the true hydrolytic capacity within the phagosomal lumen is often difficult as it is subject to many factors beyond recruitment of lysosomal hydrolases. Here we outline three assays that allow quantitative measurements of serine-cysteine protease, triglyceride lipase and β-galactosidase activities within the phagosomes of macrophages, in real time. The assays utilize ratio fluorometry between particle-associated fluorogenic substrates and calibration fluorochromes to yield internally controlled values that record rates of substrate hydrolysis. The methods described utilize a spectrofluorometer for fluorometric measurements from a population of macrophages. These assays however, could be expanded to high-throughput or single cell formats. In addition, this approach can be applied to measure a wide variety of phagosomal hydrolytic properties with the design of suitable fluorogenic substrates.
Keywords: phagosome, lysosome, phagocytosis, macrophage, hydrolase, lipase, proteolysis, lumen
1. Introduction
The ability of the macrophage to efficiently degrade phagocytosed particles is essential to its function as a mediator of homeostasis and immunity. Whilst recruitment of individual hydrolases to the phagosome has been documented extensively both biochemically and by immunofluorescence (1-3), the true hydrolytic status of the intralumenal milieu of the phagosome is only now being assessed (4). Delivery of degradative enzymes to the phagosome is a necessary first step for the development of hydrolytic activities within the organelle, but it is the complex interplay with the physiology of the phagosome that determines the actual hydrolytic activity of the phagosome. Factors such as pH, ion concentrations, redox potential, activation of proenzymes and the regulation by cofactors all impact on intraphagosomal hydrolytic activities (4). It is this complexity that precludes an accurate assessment of true phagosomal enzymatic activities based solely on the localization, or even concentration, of recruited hydrolases. In this chapter, approaches that quantify specific hydrolytic activities within the phagosome in real time, in live macrophages, are detailed.
The assays measure rates of hydrolase activities in real time, exploiting ratio fluorometry of phagocytosed, experimental particles complexed with a fluorogenic substrate and a calibration fluor. Three hydrolytic assays are described that record the degradation of fluorogenic substrates specific for phagosomal cysteine-serine proteases, triglyceride lipases and β-galactosidases. This approach should not be regarded as limited to the substrates described. Fluorogenic substrates that are pH-insensitive and can be coupled to an experimental particle could readily be used to measure a specific hydrolytic activity of interest. The activities of nucleases, phosphatases, disulfide reductases and of specific cathepsins, to name a few, could be measured using this approach with the design of suitable substrates.
The assays described are population-based experiments that follow the synchronous uptake of experimental particles by a monolayer of macrophages. This is a convenient and efficient approach as it returns statistically robust data with excellent temporal resolution. In addition, the influence of operator bias is virtually eliminated as minimal qualitative assessment is required. Furthermore, although not documented here, these methodologies could be applied to confocal-based technologies to permit recording of single cell or single phagosome measurements, and can thus assess the variation between individual phagosomes (5).
This Chapter is intended as a companion to a Chapter entitled “Real-time spectrofluorometric assays for the lumenal environment of the maturing phagosome” published in Humana Press Methods in Molecular Biology series volume X.
2. Materials
2.1 Cells, reagents and buffers
Macrophages: Both bone-marrow derived murine macrophages (BMMØ) and macrophage-like cell lines have been used (see Note 1). BMMØ’s are derived from the bone marrow extracted from the femurs, tibias and iliums of euthanized mice and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), 5% horse serum, 2 mM L-glutamine, 1 mM sodium pyruvate and 20% L-cell conditioned media (BMMØ media). RAW 264.7 cells (available from the American Type Culture Collection, Rockville, MD, USA) are maintained in DMEM supplemented with 10% FBS and 1.5 g/ml sodium bicarbonate.
Cover slips: Clean 0.13 × 12.5 × 25 mm cover glass (see Note 2). Sterilize by autoclave.
Binding buffer: Tissue culture tested PBS pH 7.2 (Gibco, Grand Island, NY, USA) adjusted to contain 1 mM CaCl2, 2.7 mM KCl, 0.5 mM MgCl2, 5 mM dextrose, 10 mM HEPES and 5 % FBS.
Cuvette buffer: Tissue culture tested PBS pH 7.2 (Gibco, Grand Island, NY, USA) adjusted to contain 1 mM CaCl2, 2.7 mM KCl, 0.5 mM MgCl2, 5 mM dextrose, 10 mM HEPES and 0.1 % calf skin gelatin (see Note 3).
Binding dish: A microbiological Petri dish containing a square piece of parafilm adhered to the lower plate surrounded by a damp Kimwipe ® tissue.
0.4 % trypan blue (Gibo, Grand Island, NY, USA).
3.0 μm carboxylate-modified silica particles (Si-COOH) 5 % suspension (Kisker Biotech, Steinfurt, Germany) (see Note 4).
Streptavidin (Promega, Madison, WI, USA). Store as desiccate at -20 °C.
Human IgG (Sigma, St Louis, MO, USA). Store as desiccate at 4 °C.
Cyanamide (Sigma, St Louis, MO, USA). Store as desiccate at 4 °C.
Coupling Buffer: 0.1 M sodium borate (Sigma, St Louis, MO, USA) in ddH2O. Adjust pH to 8.0 with 10 M NaOH. Filter sterilize through 0.22 μm filter.
Quenching Buffer: 250 mM glycine (Sigma, St Louis, MO, USA) in PBS pH 7.2. Filter sterilize through 0.22 μm filter.
Sodium azide 2 % aqueous solution. Very toxic.
-
Alexa Fluor 594® carboxylic acid, succinimidyl ester (mixed isomers) (Alexa594SE) (Molecular Probes, Eugene, OR, USA). Dissolve in high quality anhydrous dimethylsulfoxide (DMSO) (Sigma, St Louis, MO, USA) at 5 mg/ml before use.
Stock solutions can be aliquoted and stored at -20°C. Protect from light and moisture.
Biotinylated cysteine-serine protease substrate: (Biotin-LC-Phe-Arg)2-Rhodamine 110 (AnaSpec Inc., San Jose, CA, USA). Dissolve in high quality anhydrous dimethylsulfoxide (DMSO) (Sigma, St Louis, MO, USA) at 1 mg/ml before use. Stock solutions can be aliquoted and stored at -20°C. Protect from light and moisture.
Nucleosil 120-3 C18 3μm silica core reverse phase HPLC matrix (Macherey-Nagel, Easton, PA, USA). 10 mg/ml suspension in chloroform.
Triglyceride lipase substrate: 1-trinitrophenyl-amino-dodecanoyl-2pyrenedecanoyl-3-O-hexadecyl-sn-glycerol (donated by Albin Hermetter, Graz University of Technology, Graz, Austria). Dissolve in 2:1 choloroform: methanol at 1mg/ml and store at -20°C under nitrogen. Protect from light and moisture.
1-palmitoyl-2oleoyl-sn-glycero-3-phospho-rac-(1-glycerol) (Sigma, St Louis, MO, USA). Dissolve in 2:1 choloroform: methanol at 10 mg/ml and store at -20°C under nitrogen.
Octadecyl rhodamine B chloride (Molecular Probes, Eugene, OR, USA). Dissolve in methanol at 1 mg/ml and store at -20°C under nitrogen.
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(Cap Biotinyl) (Avanti Polar Lipids, Inc., Alabaster, AL, USA). Dissolve in 2:1 choloroform: methanol at 1 mg/ml and store at -20°C under nitrogen.
Cholesterol (Sigma St Louis, MO, USA). Dissolve in 2:1 choloroform: methanol at 1 mg/ml and store at -20°C under nitrogen.
Mouse monoclonal (clone BN-34) anti-biotin IgG as ascites fluid (Sigma St Louis, MO, USA).
β-Galactosidase substrate: 5-dodecanoylaminofluorescein di-β-D-galactopyranoside (C12FDG) (Molecular Probes, Eugene, OR, USA). Dissolve in 2:1 choloroform: methanol at 10 mg/ml and store at -20°C under nitrogen. Protect from light and moisture.
2.2 Instruments
Temperature controlled spectrofluorometer with variable excitation and emission monochromators. The authors use the QMSE4 model spectrofluorometer from Photon Technologies International (Lawrenceville, NJ, USA) equipped with a thermostat-controlled 4 chambered turret for simultaneous measurement of 4 experimental variables. The QMSE4 is interfaced with a PC compatible computer and is managed by Felix32 software (Photon Technologies International, Lawrenceville, NJ, USA).
Quartz 10×10×45 mm cuvettes (Fisher Scientific, Pittsburgh, PA, USA).
Fluorescent microscope with standard FITC and Texas Red filter sets. The authors use the Zeiss Axioskop 2 plus (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA).
3. Methods
3.1 Macrophage monolayer preparation and handling
Fully differentiated BMMØ monolayers are grown to confluency in untreated Petri dishes. Growth media is removed and replaced with cold PBS pH 7.2 (without Ca2+ and Mg2+) and incubated at 4 °C for 10 minutes to facilitate BMMØ detachment from the plastic. BMMØ’s are then gently dislodged with a rubber policeman and centrifuged at 230 g at 4 °C for 10 minutes.
Sterile, clean 12.5 × 25 mm cover slips are placed in a sterile Petri dish using fine-point forceps that have been dipped in 70 % ethanol and flamed (see Note 5).
BMMØ’s are gently resuspended in 1 ml BMMØ media, counted using a hemocytometer and diluted appropriately in BMMØ media to achieve ~1.25 × 106 macrophages/ml.
10 ml of BMMØ suspension is added to the Petri dish and incubated at 37 °C for 24 hours to allow a monolayer to establish on the cover slips. Care should be exercised to prevent excessive movement of the cover slips in the Petri dish. The BMMØ-monolayer covered cover slips (subsequently referred to as monolayers) are then ready for fluorometric analysis.
3.2 Spectrofluorometer setup and operation
The spectrofluorometer should be set up according to manufacturer’s directions such that optimal measurements can be taken using the desired wavelengths (see Note 6).
Clean quartz cuvettes containing cuvette buffer are inserted into a thermostat-controlled sample holder and warmed to 37 °C prior to the loading of the monolayers (see Note 7).
Using fine-point forceps, a monolayer-covered cover slip is grasped at one end (see Note 8) and dipped 10 times into a sterile 50 ml tube containing binding buffer to remove BMMØ growth media and loosely adhered cells. The cover slip is then placed in the cuvette with a vertical orientation (length of the cover slip parallel to the long axis of the cuvette), on the diagonal (width of the cover slip at 45° to the short axes of the cuvette) and with the cellular side facing the emission slit, as shown in Fig. 1.
At this point, sufficient background measurements of each monolayer are recorded for the wavelengths required.
At the conclusion of the background determination, the cover slips are carefully removed from the cuvettes using forceps and placed with the cellular side up, on the parafilm within the binding dish.
85 μl of a suspension of the appropriate experimental particles is carefully laid over each monolayer. The meniscus should be maintained over the cover slip at room temperature for 3 minutes (see Note 9).
The cover slips are once again dipped 10 times in binding buffer to remove unbound particles and are placed in the cuvettes with the same orientation.
The appropriate successive fluorescent measurements are recorded, alternating between each sample.
At the conclusion of the assay, the cover slips are removed by forceps and placed cellular side down onto 30 μl of 0.4 % trypan blue on a glass slide and examined with bright-field and fluorescence microscopy. Careful attention should be paid to macrophage viability, optimal bead to BMMØ ratio and the presence of extracellular experimental particles. This is an extremely important control and must be completed with every sample. Data should be disregarded should there be a decrease in macrophage viability, overloaded macrophages or the presence of any extracellular particles.
Fig. 1.
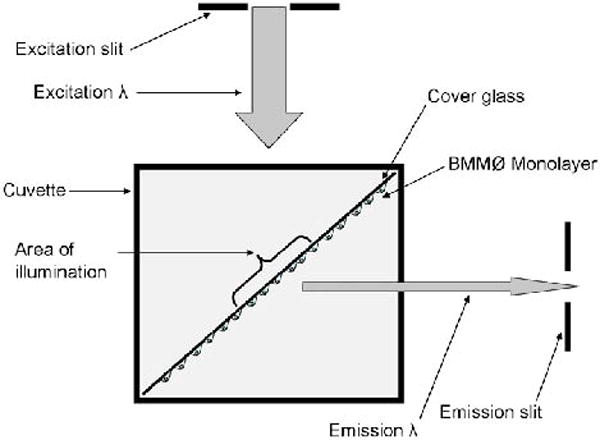
Orientation of the BMMØ monolayer in the spectrofluorometer. Cover glass is oriented to achieve a 45° incidence with the excitation beam (excitation λ), and with the cells facing the emission slit.
3.3. Kinetic analysis of intraphagosomal cysteine-serine protease activity
3.3.1. Preparation of IgG/streptavidin-coupled particles
50 mg of carboxylate-modified silica particles is washed three times in 1 ml of PBS by brief vortexing and centrifugation in a tabletop microfuge at 2000 g for 60 seconds.
Particles are resuspended in PBS (pH 7.2) with 25 mg/ml of the heterobifunctional crosslinker cyanamide (freshly made) and incubated at room temperature with agitation for 15 minutes. Excess cyanamide is removed by washing the particles twice with coupling buffer.
Particles are resuspended in 500 μl of coupling buffer with 1mg of streptavidin and 250 μg of human IgG, and incubated with agitation for 12 hours. This step covalently attaches the streptavidin and IgG to the particles through the cyanamide crosslinker. The IgG serves as an opsonin whilst the streptavidin allows coupling of the biotinylated substrate onto the particle (see Note 10).
Particles are washed twice with quenching buffer to quench unreacted cyanamide and twice with coupling buffer to remove soluble amine groups.
The particles are resuspended in 1 ml of coupling buffer, and 10 μl of the 5 mg/ml stock of the calibration fluor (Alexa Fluor 594 ® -SE) in DMSO is added (see Note 11). Incubation at room temperature with agitation for 1 hour allows the particle-bound albumin to be sufficiently labeled with the amine-reactive fluor.
The now fluorescent particles are washed three times with quenching buffer and finally resuspended in 1 ml PBS (see Note 12). 10 μl of 2 % solution of the preservative sodium azide can be added for storage at 4 °C.
3.3.2. Fluorometric measurement of phagosomal cysteine/serine protease activity
50 μl of IgG/streptavidin particles are washed twice with 500 μl sterile PBS to remove traces of sodium azide and resuspended in 80 μl of PBS.
20 μl of the 1mg/ml solution of the substrate (Biotin-LC-Phe-Arg)2-Rhodamine 110 in DMSO is added to the washed beads and incubated in the dark, on ice, for 1 hour with occasional gentle vortexing.
The now substrate-loaded particles are washed twice with 500 μl sterile PBS to remove unbound substrate and resuspended in an appropriate volume of binding buffer to achieve ~ 1 × 107 beads/ml (see Note 13).
After 594∣620 nm and 490∣515 nm (excitation λ ∣ emission λ) background measurements are recorded for BMMØ monolayers alone, 85 μl of the diluted particle suspension is laid onto the monolayers in the binding dish and incubated at room temperature for 3 minutes (as per section 3.2).
Unbound particles are washed from the monolayers by dipping in binding buffer and returned to the spectrofluorometer.
Both calibration and substrate fluorescent intensities are measured. The fluorescence of the calibration fluor Alexa Fluor 594 ® (emission at 620 nm with excitation at 594 nm) should remain constant throughout the assay (see Note 14). The rhodamine 110 fluorescence (emission at 515 nm with excitation at 490 nm) will increase as it is dequenched with sequential cleavage of the amine-linked peptides by cysteine or serine proteases, particularly cathepsin L (6). Typically an integration time of 1 second per data point is optimal. Data is collected for at least 60 minutes or until exhaustion of the substrate (see Note 15). At the conclusion of the assay, monolayers are examined via microscopy with trypan blue (as per section 3.2).
Data is exported into a standard spreadsheet application such as Microsoft Excel ®.
The appropriate background values are deducted (if not already deducted by the acquisition software) and the ratio SRT/C (where SRT=substrate fluorescence in real time and C=calibration fluorescence) is plotted against time (Fig 2).
Gradients of portions of the profiles will give relative values of the cysteine and serine protease activities of the phagosomal population over that period.
Fig. 2.
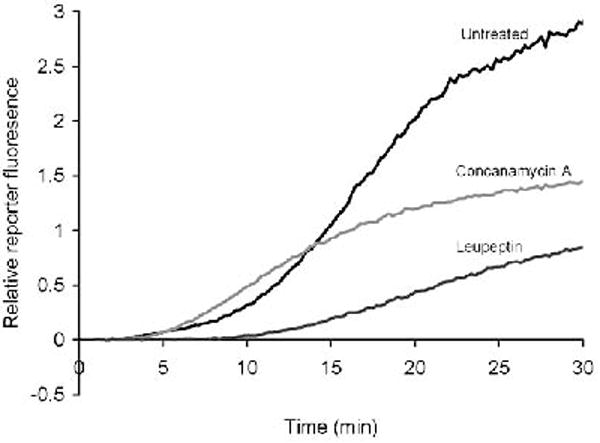
Phagosomal serine-cysteine protease activity in BMMØ’s. Serine-cysteine protease activity can be diminished with the serine-cysteine protease inhibitor leupeptin (100 μg/ml) and the V-ATPase inhibitor concanamycin A (100 nm).
3.4. Kinetic analysis of intraphagosomal triglyceride lipase activity
3.4.1. Preparation of lipase lipid monolayer particles
200 μl of the 10mg/ml suspension of the Nucleosil 120-3 C18 reverse phase HPLC matrix in chloroform is added to a clean glass test tube along with the following lipids dissolved in 2:1 chloroform: methanol (see Note 16): 25 μl of the substrate 1-trinitrophenyl-amino-dodecanoyl-2-pyrenedecanoyl-3-O-hexadecyl-sn-glycerol (1mg/ml); 5 μl of the calibration fluor octadecyl rhodamine B chloride (1mg/ml); 30 μl of the phospholipid 1-palmitoyl-2oleoyl-sn-glycero-3-phospho-rac-(1 glycerol); 5 μl of the biotinylated phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(Cap Biotinyl) (1mg/ml); 5 μl of cholesterol (10mg/ml).
The mixture is briefly sonicated in a bath sonicator at 40°C and then dried under nitrogen (see Note 17).
The test tube is transferred to the bath sonicator at 40°C and 500 μl of sterile PBS is added to the tube during sonication. This facilitates the arrangement of the amphipathic lipids to surround the hydrophobic C18-coupled particles, allowing the particles to be miscible in the PBS. The mixture is sonicated until a homogenous suspension is achieved (see Note 18).
The suspension is cooled immediately by placing the test tube on ice. To avoid disruption of the lipid monolayer on the surface of the particles, which results in clumping of the beads, the suspension must be kept ice cold during subsequent manipulation and not be subjected to excessive shearing or centrifugal forces.
Once cooled, the suspension is transferred to 1.5 ml polypropylene tubes and the lipid particles are washed twice with 500 μl ice cold PBS using gentle centrifugation at 100 g for no more than 30 seconds.
Lipid particles are resuspended in 100 μl PBS to which 5μl of anti-biotin IgGcontaining mouse ascites fluid is added. Incubation on ice for 30 minutes with occasional gentle agitation is sufficient to permit IgG-opsonization of the particles.
The opsonized lipid particles are gently washed twice with 500 μl ice cold PBS to remove excess IgG and finally suspended in 500 μl of PBS (see Note 12). The particles are stable for 24 hours if kept on ice in the dark.
3.4.2. Fluorometric measurement of phagosomal triglyceride lipase activity
The lipid particles are diluted in an appropriate volume of binding buffer to achieve ~ 1 × 107 beads/ml (see Note 13).
After 555∣610 nm and 342∣400 nm (excitation λ ∣ emission λ) background measurements are recorded for BMMØ monolayers alone, 85 μl of the diluted particle suspension is laid onto the monolayers in the binding dish and incubated at room temperature for 3 minutes (as per section 3.2).
Unbound particles are washed from the monolayers by dipping in binding buffer and returned to the spectrofluorometer.
Both calibration and substrate fluorescent intensities are measured. The fluorescence of the calibration fluor octadecyl rhodamine B chloride (emission at 610 nm with excitation at 555 nm) should remain constant throughout the assay (see Note 14). The substrate fluorescence (emission at 400 nm with excitation at 342 nm) will increase with progressive esterolysis of the triglyceride analogue as it liberates the fluorescent pyrenedecanoic acid and the quenching trinitrophenylamino acyl residues from the glycerol backbone (7). Typically an integration time of 1 second per data point is optimal. Data is collected for at least 60 minutes or until exhaustion of the substrate. At the conclusion of the assay, monolayers are examined via microscopy with trypan blue (as per section 3.2).
Data is exported into a standard spreadsheet application such as Microsoft Excel ®.
The appropriate background values are deducted (if not already deducted by the acquisition software) and the ratio SRT/C (where SRT=substrate fluorescence in real time and C=calibration fluorescence) is plotted against time (Fig 3).
Gradients of portions of the profiles will give relative values of the triglyceride lipase activity of the phagosomal population over that period.
Fig. 3.
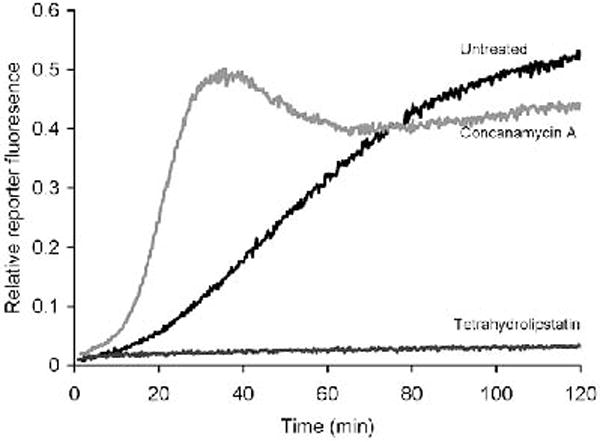
Phagosomal triglyceride lipase activity in BMMØ’s. Lipase activity can be diminished with the specific lipase inhibitor tetrahydrolipstatin (50 μg/ml) and is increased in the early phagosome by the V-ATPase inhibitor concanamycin A (100 nm).
3.5. Kinetic analysis of intraphagosomal β-galactosidase activity
3.5.1. Preparation of β-galactosidase lipid monolayer particles
The lipid monolayer particle is used as the carrier for the β-galactoside substrate and is essentially prepared as described for the lipase lipid monolayer particles in section 3.4.1. However, the lipid composition of the particles is modified to allow for a greater proportion of the β-galactosidase substrate. To 200 μl of the 10mg/ml suspension of the Nucleosil 120-3 C18 reverse phase HPLC matrix in chloroform the following lipids are added: 10 μl of the substrate 5-dodecanoylaminofluorescein-di-β-D-galactopyranoside (C12FDG) (10mg/ml); 5 μl of the calibration fluor octadecyl rhodamine B chloride (1mg/ml); 20 μl of the phospholipid 1-palmitoyl-2oleoyl-sn-glycero-3-phospho-rac-(1-glycerol); 5 μl of the biotinylated phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamineN-(Cap Biotinyl) (1mg/ml); 5 μl of cholesterol (10mg/ml).
The mixture is dried, resuspended in PBS and particles washed and IgG opsonised as described in section 3.4.1.
3.5.2. Fluorometric measurement of phagosomal β-galactosidase activity
The lipid particles are diluted in an appropriate volume of binding buffer to achieve ~ 1 × 107 beads/ml (see Note 13).
After 555∣610 nm and 488∣520 nm (excitation λ ∣ emission λ) background measurements are recorded for BMMØ monolayers alone, 85 μl of the diluted particle suspension is laid onto the monolayers in the binding dish and incubated at room temperature for 3 minutes (as per section 3.2).
Unbound particles are washed from the monolayers by dipping in binding buffer and returned to the spectrofluorometer.
Both calibration and substrate fluorescent intensities are measured. The fluorescence of the calibration fluor octadecyl rhodamine B chloride (emission at 610 nm with excitation at 555 nm) should remain constant throughout the assay (see Note 14). The fluorescent intensity of the fluorescein-based substrate (emission at 515 nm with excitation at 490 nm) will increase as it is dequenched with sequential cleavage of the two galactopyranosides (see Note 19). Typically an integration time of 1 second per data point is optimal. Data is collected for at least 90 minutes or until exhaustion of the substrate. At the conclusion of the assay, monolayers are examined via microscopy with trypan blue (as per section 3.2).
Data is exported into a standard spreadsheet application such as Microsoft Excel ®.
The appropriate background values are deducted (if not already deducted by the acquisition software) and the ratio SRT/C (where SRT=substrate fluorescence in real time and C=calibration fluorescence) is plotted against time (Fig 4).
Gradients of portions of the profiles will give relative values of the βgalactosidase activity of the phagosomal population over that period.
Fig. 4.
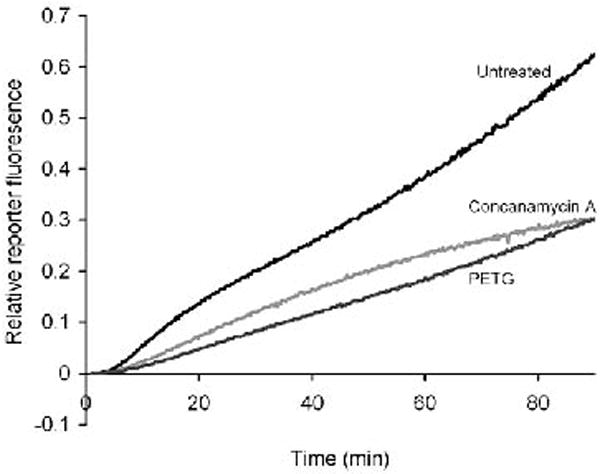
Phagosomal β-galactosidase activity in BMMØ’s. β-galactosidase activity can be diminished with the competitive inhibitor 2-phenylethyl-β-D-thiogalactoside (PETG) (200 μg/ml) and the V-ATPase inhibitor concanamycin A (100 nm).
4. Notes
Primary BMMØ’s are generally preferred for their enhanced phagocytic proficiency and adhesion.
0.13 × 12.5 × 25 mm cover glass is not commercially available. Cover glass can be custom ordered from ProSciTech (Thuringowa, Qld, Australia). Alternatively, 25 × 25 mm cover glass is available from Fisher Scientific (Pittsburgh, PA, USA) and can be cut in half by diamond pencil in house.
FBS is substituted for gelatin for spectrofluometric assays as it has low autofluorescence with excitation wavelengths above 450 nm. If assays are expected to take greater than 6 hours, FBS is preferred for sustained macrophage viability.
The authors prefer the 3.0 μm −COOH-modified silica particles due to their ease of handling and low autofluorescence, however polystyrene particles and particles of smaller size can be used successfully. Generally, macrophage-like cell lines do not efficiently phagocytose particles with diameters over 2.0 μm. Therefore particles >2.0 μm are not advised for those cell types.
Arrange cover slips so as not to overlap, taking care not to overcrowd them in the Petri dish as cover slips can move after monolayers have been established and damage to BMMØ’s can occur. Alternatively, cover slips can be separated from each other using partitioned Petri dishes or 6 well plates.
Some general considerations are: the focusing of illumination on sample, the addition of long-pass and short-pass filters, and the adjustment of excitation and emission slit width to maximize signal to noise ratio and to minimize photobleaching.
Cuvette buffer should be of a similar temperature to the cuvettes at addition. This prevents bubble formation that can create unwanted scatter of light.
Cover slips should only be grasped by forceps at the uppermost edge to avoid damaging the area of the monolayer that is illuminated in the spectrofluorometer.
Binding time may need to be increased if smaller particles or polystyrene beads are used.
Particles can be directed through the mannose receptor by substitution of the human IgG with the equivalent amount of α-D-mannosylated-PITC-albumin (Sigma St Louis, MO, USA).
Ensure that the fluor/DMSO aliquot is fully thawed and at room temperature before opening to avoid wetting the DMSO.
Labeled particles should firstly be microscopically examined to ensure adequate fluorescence and absence of clumping.
A suitable dilution of beads should be determined for each batch. This should be done by applying the bead suspension to a BMMØ monolayer on cover glass for the desired binding time, washing off unbound beads and assessing degree of bead adhesion under magnification. For most applications a target of 1-2 beads/macrophage is desired. Due to their low density, significantly higher concentrations of polystyrene beads are needed to achieve optimal binding to monolayers.
It is preferred, but not usually necessary, to record the calibration fluorescence throughout the entire assay. Recording a calibration fluorescence at the beginning and/or conclusion of the assay will increase time resolution of reporter fluor profiles and may allow the use of filter sets that improve signal to noise ratios.
The free rhodamine 110 is moderately membrane permeable and thus will leach from the cell over time. This may result in a decrease in fluorescent intensity.
Ensure that stock aliquots are warmed to room temperature before opening to ensure that the sample remains anhydrous.
Gently swirl the test tube while under a gentle stream of nitrogen to dry the material in a film surrounding the bottom of the test tube. This will help in the proper resuspension of the particles in the sonication step.
It may be necessary to pipette the suspension up and down to help break the surface tension.
The fluorescent properties of fluorescein-based compounds are commonly sensitive to changes in pH and hence unsuitable for real-time measurements within acidifying compartments. However, once incorporated in the lipid monolayer of the experimental particle, the fluorescent intensity of the hydrolysed C12FDG is less sensitive to changes in pH (data not shown) making it more suitable for phagosomal assays.
References
- 1.Ullrich HJ, Beatty WL, Russell DG. Direct delivery of procathepsin D to phagosomes: implications for phagosome biogenesis and parasitism by Mycobacterium. Eur J Cell Biol. 1999;78:739–748. doi: 10.1016/S0171-9335(99)80042-9. [DOI] [PubMed] [Google Scholar]
- 2.Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claus V, Jahraus A, Tjelle T, Berg T, Kirschke H, Faulstich H, Griffiths G. Lysosomal enzyme trafficking between phagosomes, endosomes, and lysosomes in J774 macrophages. Enrichment of cathepsin H in early endosomes. J Biol Chem. 1998;273:9842–9851. doi: 10.1074/jbc.273.16.9842. [DOI] [PubMed] [Google Scholar]
- 4.Yates RM, Hermetter A, Russell DG. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6:413–420. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 5.Henry RM, Hoppe AD, Joshi N, Swanson JA. The uniformity of phagosome maturation in macrophages. J Cell Biol. 2004;164:185–194. doi: 10.1083/jcb.200307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assfalg-Machleidt I, Rothe G, Klingel S, Banati R, Mangel WF, Valet G, Machleidt W. Membrane permeable fluorogenic rhodamine substrates for selective determination of cathepsin L. Biol Chem Hoppe Seyler. 1992;373:433–440. doi: 10.1515/bchm3.1992.373.2.433. [DOI] [PubMed] [Google Scholar]
- 7.Duque M, Graupner M, Stutz H, Wicher I, Zechner R, Paltauf F, Hermetter A. New fluorogenic triacylglycerol analogs as substrates for the determination and chiral discrimination of lipase activities. J Lipid Res. 1996;37:868–876. [PubMed] [Google Scholar]


