Abstract
Human immunodeficiency virus type 1 (HIV-1)-specific T-cell reactivity has been related to protection from disease progression. Optimal T-cell reactivity to HIV-1 presumably requires antigen processing and presentation by professional antigen-presenting cells, particularly dendritic cells (DC). Here we examined whether multiple HIV-1-specific T-cell functions are enhanced by stimulation with HIV-1 peptide-loaded DC derived from HIV-1-infected subjects on antiretroviral therapy. We first found that mature DC increased the number of gamma interferon (IFN-γ)-producing T cells detected by enzyme-linked immunospot assay to overlapping 15-mer peptides of HIV-1 Gag and Nef, compared to stimulation with peptide-loaded, immature DC or to peptides without DC. IFN-γ production was lower in response to large pools of the Gag and Nef peptides, regardless of presentation by DC. We further observed that HIV-1 peptide-loaded, mature DC stimulated greater CD8+ and CD4+ T-cell proliferation than did the peptides without DC and that T-cell proliferation was lower in response to larger pools of the peptides. The lower T-cell IFN-γ and proliferation responses to the larger peptide pools were related to lower T-cell viability. Finally, the number of polyfunctional CD8+ and CD4+ T cells stimulated by HIV-1 peptide-loaded, mature DC, defined as positive by intracellular staining for more than one immune mediator (IFN-γ, interleukin 2, tumor necrosis factor alpha, macrophage inhibitory protein 1β, or CD107a), was greater than that stimulated by the peptides alone. These results indicate that DC can enhance multiple types of HIV-1-specific T-cell functions.
Considerable evidence supports the idea that T-cell immunity to human immunodeficiency virus type 1 (HIV-1) is important in control of HIV-1 infection (10). Specific correlates of T-cell immunity that are associated with protection against or progression of HIV-1 infection have nonetheless been difficult to determine. Such immune correlates could be useful in defining the efficacy of prophylactic and therapeutic vaccines for HIV-1 infection. Many studies of T-cell immunity in HIV-1 infection have shown that the number of T cells exhibiting gamma interferon (IFN-γ) production in the enzyme-linked immunospot (ELISPOT) assay is decreased in association with progressive infection (4, 51). Proliferation of T cells in response to HIV-1 antigens as measured by uptake of the succinimidyl ester of carboxyfluorescein diacetate (CFSE) has also been related to less progressive HIV-1 infection (19, 33, 53). Recently, the quality of the CD8+ T-cell functional response to HIV-1 peptides as defined by intracellular cytokine staining (ICS) for more than one immune mediator, i.e., IFN-γ, interleukin 2 (IL-2), tumor necrosis factor alpha (TNF-α), macrophage inhibitory protein 1β (MIP-1β), and/or cytotoxic degranulation molecule CD107a (11, 44), has been associated with slow progression and better control of HIV-1 infection (5).
Although these are all valid measures of anti-HIV-1 T-cell immunity, they usually do not account for a role of professional antigen-presenting cells (APC), particularly dendritic cells (DC), which are necessary for optimal processing and presentation of antigens to T cells (2). Indeed, it is likely that during HIV-1 infection, DC are required to take up, process, and present HIV-1 antigens via their major histocompatibility complex (MHC) class I and II molecules for priming and boosting of anti-HIV-1 CD8+ and CD4+ T-cell responses (40). We have previously shown that IFN-γ production by CD8+ T cells from HIV-1-infected persons is enhanced by stimulation with DC loaded with HIV-1 antigens and matured with CD40L or a cocktail of various proinflammatory cytokines and a Toll-like receptor 3 ligand (15, 20, 21). Myeloid DC loaded with peptides representing dominant epitopes of HIV-1 proteins stimulated significantly more epitope-specific, IFN-γ-producing CD8+ T cells than did peptides added directly to peripheral blood mononuclear cells (PBMC). There is little information, however, as to whether these professional APC can similarly enhance other T-cell functions that could be critical to control of HIV-1 infection, particularly their proliferative capacity and ability to produce multiple immune mediators. Moreover, many current approaches for measuring the magnitude and breadth of T-cell responses use pools of various numbers of synthetic peptides, usually 15 or 20 amino acids (aa) in length, which overlap by 10 to 11 aa (1, 3, 7, 9, 13, 14, 17, 24, 25, 27, 32, 37, 45, 48, 49), developed by Kern et al. (26) and Maecker et al. (31). Such studies have not accounted for a role of APC in processing that is required to reduce these peptides to their optimal, 8- to 10-mer length for presentation by MHC class I molecules to CD8+ T cells (43), or to 13- to 17-mers for presentation by MHC class II to CD4+ T cells (46). These are important considerations in determining correlates of T-cell immunity in HIV-1 infection and in response to HIV-1 vaccines.
We have analyzed the magnitude of several types of T-cell responses during HIV-1 infection stimulated by autologous DC loaded with different-size pools of overlapping HIV-1 peptides. We assessed T-cell responses in HIV-1- infected persons for single-cell IFN-γ production by using a conventional ELISPOT assay; for CD8+ and CD4+ T-cell proliferation by using uptake of CFSE dye; and for production of IFN-γ, IL-2, TNF-α, MIP-1β, and CD107a by CD8+ and CD4+ T cells by using ICS. We found that, in addition to enhancing IFN-γ production detected by ELISPOT assay, DC loaded with HIV-1 peptide singlets or smaller pools of HIV-1 peptides enhanced HIV-1-specific T-cell proliferation and polyfunctional CD8+ and CD4+ T-cell responses.
MATERIALS AND METHODS
Study subjects.
Ten HIV-1-seropositive homosexual men were studied from the Pittsburgh, PA, portion of the Multicenter AIDS Cohort Study, an investigation of the natural history of HIV infection. Ten HIV-1-seropositive subjects were all on antiretroviral therapy (ART) (CD4+ T-cell count: median, 501/μl; range, 340 to 1,110/μl; HIV-1 RNA: <40 copies/ml for nine subjects and 9,244 copies/ml for one subject. Five HIV-1-seronegative persons were included as controls.
DC cultures.
To obtain immature DC, CD14+ monocytes were positively selected from PBMC using anti-CD14 monoclonal antibody (MAb)-coated magnetic microbeads (Stem Cell Technologies, Vancouver, Canada) to a purity of >96% and cultured for 5 to 6 days at 37°C in AIM V medium (Gibco, Grand Island, NY) containing 1,000 U/ml of recombinant human IL-4 (R & D Systems, Minneapolis, MN) and 1,000 U/ml recombinant granulocyte-monocyte colony-stimulating factor (Amgen, Seattle, WA) (21). Fresh medium with human IL-4 and granulocyte-monocyte colony-stimulating factor was added every other day. These immature DC were treated with CD40L (0.5 μg/ml; Alexis, San Diego, CA) for 40 h to induce DC maturation.
The number of viable DC was determined by typical DC morphology in trypan blue dye-stained preparations. The maturation status of the DC was assessed by flow cytometry as the percent positive and mean fluorescent intensity of expression of HLA class II (HLA-DR), HLA class I (HLA ABC), CD80, CD86, and CD83 (15, 21). The cells displayed a characteristic DC morphology and cell surface marker expression and responded to stimulation with CD40L.
Synthetic peptides.
A library of HIV-1 peptides (consecutive 15-mers overlapping by 11 aa) spanning the HIV-1 Gag and Nef (clade B consensus sequences) was obtained from the NIH AIDS Research and Reference Reagent Repository and distributed into various-sized pools ranging from 9 to 122 peptides. The Gag pools were Gag A30 (30 p17 15-mer peptides; aa 1 to 131), B30 (30 p24 peptides; aa 117 to 251), C30 (30 p24 peptides; aa 241 to 371), D32 (32 p24, p2, and p7 peptides; aa 361 to 500), Gag E60 (60 peptides of p17 and p24), F62 (62 peptides of p24, p2, and p7), and Gag G122 (122 peptides of p17, p24, p2, and p7). Nef pools were A10 (10 15-mer peptides; aa 1 to 51), B10 (10 peptides; aa 41 to 91), C10 (10 peptides; aa 81 to 131), D10 (10 peptides; aa 21 to 170), E9 (nine peptides; aa 161 to 207), Nef F25 (25 peptides; aa 1 to 111), Nef F24 (24 peptides; aa 101 to 207), and Nef H49 (49 peptides; aa 1 to 207). The final peptide concentration was adjusted to 5 μg/ml in singlets or pools. Peptides included HLA A*0201-restricted, Epstein-Barr virus (EBV) BMLF1280-288 (GL9; GLCTLVAML), its N-terminal flanking sequence (AL15; AIQNAG-L), and its C-terminal flanking sequence (GW15; G-LEETIFW). HIV-1 p1777-85 (SL9; SLYNTVATL), 15-mers of its N-terminal flanking sequence (GL15; GSEELRS-L) and C-terminal flanking sequence (SR15; S-LYCVHQR), the 15-mer from the NIH Gag peptide library containing SL9 (EC15; EELRSLYNTVATLYC), and a 20-mer containing SL9 (GQ20; GSEELRSLYNTVATLYCVHQ) were also used. Other peptides were HLA B27-restricted HIV-1 p24263-272 (KK10; KRWIILGLNK) and HLA A*0201-restricted HIV-1 peptide RT476-484 (IV9; ILKEPVHGV). All of these peptides were prepared by the Protein Research Lab, University of Illinois, Chicago, IL. A pool of four cytomegalovirus, 15 EBV, and 12 influenza virus peptides representing a cross section of MHC class I dominant epitopes (8) was provided by the NIH AIDS Research and Reference Reagent Program. Preliminary dose- response experiments were done to determine the optimal concentration of peptides to be used in the functional assays.
ELISPOT assay.
An ELISPOT assay modified from AIDS Clinical Trials Group protocol A5181 was used to determine single-cell IFN-γ production (B. J. C. Macatangay et al., unpublished data). The immature or mature DC were added to ELISPOT plates and loaded with peptides (5 μg/ml) for 2 h at 37°C. The responder cells (PBMC) were added to the peptide-loaded DC at a responder-to-stimulator [R:S] cell ratio of 10 to 1 and stimulated overnight at 37°C. Negative control (without peptides), positive control (chicken embryo fibroblasts, 1 μg/ml), and staphylococcus enterotoxin B (0.5 μg/ml; Sigma, St. Louis, MO) were included in each assay. After the plates were processed for staining of IFN-γ, the spots were counted with an ELISPOT reader system (Cell Technology, Columbia, MD). Data were expressed as spot-forming cells (SFC) per 106 cells. A positive result was defined as the number of SFC in the peptide-stimulated cultures being greater than the mean plus 2 standard deviations of SFC in the negative controls.
For comparison of peptide loading of DC before and after CD40L treatment for induction of IFN-γ, DC from HIV-1-uninfected and -infected subjects were loaded with peptides for 2 h before and after CD40L treatment and used to induce IFN-γ production in autologous PBMC. To determine competition by peptide pools, HLA A*0201-restricted EBV GL9, AL15, and GW15 peptides were mixed with singlets or pools of HIV-1 Gag peptides and used to stimulate IFN-γ production in PBMC from HLA A*0201, HIV-1-seronegative, EBV-seropositive subjects in the ELISPOT assay.
Lymphocyte proliferation CFSE assay.
Frozen-thawed cells (1 × 106 cells/antigen stimulation or unstimulated controls) were washed and resuspended in 250 μl of 0.2% bovine serum albumin-phosphate-buffered saline. Two hundred fifty microliters of CFSE dye (10 μg CFSE in 1 ml of 0.2% bovine serum albumin-phosphate-buffered saline; Molecular Probes, Eugene, OR) was incubated with the cell suspension at 37°C for 10 min. After incubation, 5 ml of cold complete medium was added and incubated on ice for 5 min. Cells were washed with cold RPMI 1640 medium (Gibco, Grand Island, NY) containing 10% heat-inactivated human AB+ serum (Sigma), 1% l-glutamine, 1% HEPES buffer, and 1% penicillin-streptomycin and cultured with peptides (5 μg/ml) with or without DC in a 24-well plate for 6 days at 37°C in 5% CO2. The responder cells (PBMC) were mixed with peptide-loaded (5 μg/ml) DC at a ratio of 10 to 1, R:S cell ratio. After 6 days of incubation, the cells were harvested, washed, and stained for surface markers by using anti-CD8-peridinin chlorophyll protein and anti-CD4-phycoerythrin (PE) MAb (BD Biosciences). Following staining, the cells were washed, fixed, and analyzed in a FACS Canto II flow cytometer (BD Immunocytometry Systems, San Diego, CA). Negative controls (no peptides) and positive controls (phytohemagglutinin, 5 μg/ml; Sigma) were included in each assay. The results are expressed as net % CFSE-positive T cells (% positive peptide-stimulated T cells − % medium control).
Surface staining and ICS.
Frozen-thawed PBMC at 2 × 106/ml in RPMI 1640 medium containing antibiotics and 10% heat-inactivated fetal calf serum (Gemini Bio-Products, West Sacramento, CA) (complete medium) were rested overnight at 37°C. PBMC were then cultured with T-cell costimulatory MAb specific for CD28 and CD49d (αCD28/49d) (1 μg/ml; BD Biosciences), monensin (5 μg/ml; Sigma), brefeldin A (5 μg/ml, Sigma), CD107a-PE-Cy5 (BD PharMingen, San Diego, CA), and peptides or peptide pools (5 μg/ml per peptide) (29). In some experiments, peptide-loaded DC (R:S ratio, 10:1) were used without αCD28/49d. Negative controls (without peptides) and positive controls (staphylococcal enterotoxin B, 1 μg/ml) were included in each assay. Cells were incubated for 6 h at 37°C and then kept at 4°C for 16 h. The cells were washed, fixed using the Cytofix/Cytoperm kit (BD PharMingen), and stained with MAb CD8-allophycocyanin Cy7, CD4-allophycocyanin Cy7, and IL-2-allophycocyanin (BD Biosciences); CD3-PE Cy7, IFN-γ-fluorescein isothiocyanate and MIP-1β-PE (BD PharMingen); and TNF-α-Pacific blue (eBiosciences). Following staining, the cells were washed, fixed, and analyzed with an LSR II flow cytometer (BD Immunocytometry Systems), with 200,000 to 1,000,000 events collected per sample. All data were background subtracted using the non-antigen-stimulated control and analyzed by FlowJo (version 7.2.5; TreeStar, Ashland, OR) and SPICE (version 4.1.6). The expression of IL-2, IFN-γ, TNF-α, MIP-1β, and CD107a and that of T-cell surface markers were quantified separately and in combination.
Statistical analysis.
We used the analysis of variance with the Scheffe multiple comparison test, the Student t test, and the chi-square test for statistical analysis.
RESULTS
Single-cell IFN-γ production stimulated by DC loaded with HIV-1 peptides before and after maturation.
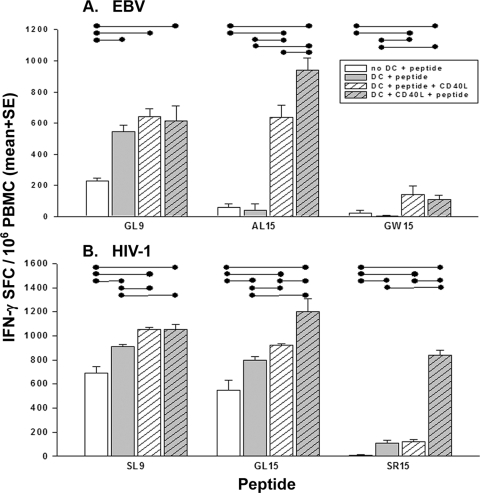
Our previous research showed that CD40L-matured DC loaded with minimal-length, immunodominant 9-mer peptides, or 15-mers with either N- or C-terminal extensions, were superior to immature DC at stimulating T-cell production of IFN-γ assessed by the ELISPOT assay (20, 21). In the present study, we extended this work by examining stimulation of T cells by CD40L-matured DC that were loaded before or after maturation with 15-mers containing N- or C-terminal extensions. We found that T-cell responses induced by the DC that had been loaded with 9-mer peptide EBV GL9 or HIV-1 SL9 before or after maturation with CD40L were similar but were both greater than T-cell responses induced by peptides alone or peptide-loaded immature DC (Fig. 1). However, using 15-mer, N-terminal extensions of these two epitopes, we noted that adding peptide after maturation of the DC with CD40L enhanced T-cell reactivity. This enhancing effect of CD40L-matured DC on T-cell responses to 15-mers was also observed with C-terminal extensions of SL9 but not GL9. Therefore, in all further experiments we used DC loaded with the peptides after maturation for stimulation of T-cell responses.
FIG. 1.
Maturation of DC enhances their ability to induce IFN-γ production by T cells as detected by the ELISPOT assay. Data shown are means ± standard errors from four HIV-1-uninfected subjects (A) and four HIV-1-infected subjects on ART (<50 copies/ml of HIV-1 RNA) (B), using HLA A*0201-restricted 9-mer peptide GL9 (A) or SL9 (B) or N- or C-terminal flanking sequences containing these 9-mer peptides. The GL9 and SL9 loaded before (DC + peptide + CD40L) or after (DC + CD40L + peptide) maturation with CD40L stimulated higher levels of IFN-γ production than did the peptide without DC (no DC + peptide) or DC loaded with peptide without CD40L treatment (DC + peptide) (•—•, P < 0.05). IFN-γ production by T cells stimulated by DC loaded with N- or C-terminal flanking sequences was greater than production by those induced by peptides alone or immature DC and the peptides (P < 0.05, except for GW15).
Enhanced single-cell IFN-γ production stimulated by DC loaded with HIV-1 peptides and peptide pools.
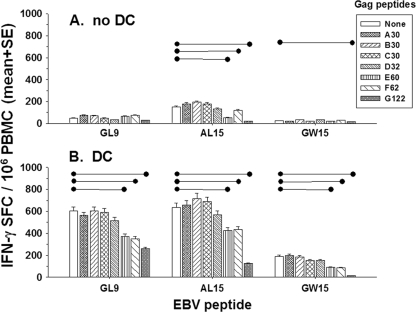
There is little information on the magnitude of T-cell reactivity to DC loaded with various-sized pools of overlapping HIV-1 peptides compared to single peptides within each pool. We therefore compared the conventional method of direct stimulation of PBMC with single peptides or different-sized peptide pools to DC that were loaded with these peptides or peptide pools in eight HIV-1-infected subjects on ART. For Gag, we compared the complete pool of 122 HIV-1 Gag 15-mer peptides (G122) to two pools of 60 to 62 peptides (60/62; E60 and F62), four pools of 30 to 32 peptides (30/32; A30, B30, C30, and D32), and the 122 individual Gag peptides. For Nef, we compared the complete pool of 49 Nef 15-mer overlapping peptides (H49), to two pools of 24 to 25 peptides (25/24; F25 and G24), five pools of 9 to 10 peptides (10/9; A10, B10, C10, D10, and E9), and the 49 individual Nef peptides.
The results show that Gag and Nef peptide-loaded DC induced higher levels of peptide-specific IFN-γ production in PBMC than did the peptides without DC (P < 0.05), except for the Gag G122 pool (P = not significant [NS]) (Fig. 2A and B). The compiled results indicate that single 15-mer peptides of Gag or Nef stimulated greater HIV-1-specific IFN-γ production than did the Gag and Nef peptide pools, with or without DC (P < 0.01). Moreover, DC loaded with the Gag 30/32 pools stimulated higher HIV-1-specific IFN-γ production than did DC loaded with Gag 60/62 or Gag 122 pools, and the Gag 60/62 pools stimulated greater T-cell reactivity than did the Gag G122 pool (P < 0.05) (Fig. 2A). Similarly, DC loaded with Nef 10/9 or Nef 25/24 pools stimulated higher HIV-1-specific IFN-γ production than did the Nef H49 pool (P < 0.05) (Fig. 2B). Finally, we found no T-cell reactivity to the Gag or Nef peptide pools from three HIV-1-seronegative controls (data not shown).
FIG. 2.
DC enhance IFN-γ production to single peptides or pools of Gag and Nef 15-mer peptides, with decreased responses to larger pools of peptides. The optimal size of pools for ELISPOT without mature DC was compared to that for mature DC loaded with single peptides or different sizes of peptide pools in HIV-1-infected subjects on ART (n = 7 subjects with <50 copies/ml of HIV-1 RNA, 1 subject with 9,244 copies/ml). Bars represent cumulative data of SFC per million PBMC for single peptides or the peptide pools. The cumulative ELISPOT responses showed that CD40L-treated DC induced higher levels of Gag (A) and Nef (B) peptide-specific IFN-γ production by PBMC than that in PBMC alone (no DC) (•—•, P < 0.05), except for the Gag G122 pool (P = NS). With or without DC, Gag or Nef single peptides stimulated greater HIV-1-specific IFN-γ production than did the Gag or Nef peptide pools (P < 0.01). DC loaded with the Gag 30/32 pool stimulated higher HIV-1-specific IFN-γ production than did the Gag 60/62 or Gag G122 pools, and the Gag 60/62 pools stimulated greater T-cell reactivity than did the Gag G122 pool (P < 0.05) (A). DC loaded with Nef 10/9 and Nef 25/24 pools stimulated higher HIV-1-specific IFN-γ production than did the Nef H49 pool (P < 0.05) (B).
Taken together, the results show that the numbers of IFN-γ-producing T cells in HIV-1-infected subjects as assessed by the ELISPOT assay were lower in response to direct stimulation with pools of Gag or Nef peptides than those stimulated by mature DC loaded with the peptide pools. This extends our previous finding that DC enhanced IFN-γ production in T cells to immunodominant 9- to 10-mer HIV-1 peptides (20, 21). Furthermore, we found that lower T-cell reactivity was induced by larger pools of overlapping Gag and Nef peptides, with or without DC.
Effects of larger peptide pools on T-cell stimulation.
We next determined if lower cell viability or competition among the viral peptides was related to the lower number of IFN-γ-producing T cells induced by the larger peptide pools. First, we determined the number of viable cells in the peptide-stimulated cultures by trypan blue dye exclusion. Compared to the viability of cells in the PBMC control not stimulated with peptides (92% ± 6%, n = 6), the viability was lower in the cultures stimulated by the larger peptide pools (84% ± 11% for Gag 30/32, P = NS; 65% ± 11% for Gag 60/62, P < 0.05; 59% ± 10% for Gag G122, P < 0.01; 88% ± 5% for Nef 10/9, P = NS; 81% ± 10% for Nef 25/24, P = NS; 70% ± 13% for Nef H49, P < 0.05).
Next, we assessed the capacity of HIV-1 Gag peptides to interfere or compete with T-cell reactivity of EBV-seropositive, HIV-1-seronegative, HLA A*0201 subjects to peptides representing a known immunodominant, HLA A*0201 epitope of EBV. We mixed HLA A*0201-restricted EBV GL9 (5 μg/ml) with low (1 μg/ml) or high (10 μg/ml) concentrations of HLA A*0201-restricted, HIV-1 peptides IV9 and SL9 or HLA B27-restricted HIV-1 peptide KK10. These peptide mixtures were then loaded into DC from three HLA A*0201, EBV-positive and HIV-1-negative subjects and used to stimulate autologous T cells in the ELISPOT assay. The results indicate that there was no difference in GL9-specific IFN-γ production when this peptide was mixed at the two concentrations with the 9-mer HIV-1 peptides (P = NS; data not shown). We next mixed EBV GL9 or its 15-mer N- and C-terminal extensions AL15 and GW15 with the different sizes of HIV-1 Gag 15-mer peptide pools, loaded into DC and used in the ELISPOT assay with PBMC from three HLA A*0201, EBV-positive, HIV-1-negative persons. The data show that there were similar T-cell responses to GL9 and AL15 without DC but lower IFN-γ production in response to GW15 (Fig. 3). This concurs with our previous results that C-terminal extensions of some immunodominant epitopes have lower capacity to stimulate IFN-γ production than the minimal epitope or N-terminal-extended peptides (21). The results further indicate that there was no difference in GL9-specific IFN-γ production when this peptide was mixed with any of the Gag peptide pools without DC (P = NS) (Fig. 3A). There were, however, decreases in AL15-specific IFN-γ production by T cells when this peptide was mixed with the Gag 60/62 pools (P < 0.05) and the Gag G122 pool (P < 0.001) and in GW15-specific IFN-γ production when this peptide was mixed with the Gag G122 pool (P < 0.05) (Fig. 3A). With the use of peptide-loaded DC as APC, there were significant decreases in GL9-, AL15-, and GW15-specific IFN-γ production when these EBV peptides were mixed with Gag 60/62 pools or the Gag G122 pool (P < 0.01 and P < 0.001, respectively; Fig. 3B). However, with or without DC, there were no differences in EBV peptide-specific IFN-γ production when these peptides were mixed with Gag 30/32 pools (P = NS).
FIG. 3.
Larger pools of HIV-1 peptides result in lower IFN-γ production by EBV-specific T cells. HLA A*0201-restricted EBV GL9 and its N (AL15)- and C (GW15)-terminal extensions (5 μg/ml) were mixed with different sizes of HIV-1 Gag peptide pools (5 μg/ml), with and without DC, in the ELISPOT assay. Data are mean SFC ± standard errors from three HIV-1-negative subjects. The data show that without DC (A) there was no difference in GL9 peptide-specific IFN-γ production when it was mixed with any of the Gag peptide pools (P = NS). There were decreases in AL15-specific IFN-γ production when this peptide was mixed with either the Gag 60/62 pool (•—•, P < 0.05) or the Gag G122 pool (•—•, P < 0.001), and there were decreases in GW15-specific IFN-γ production when this peptide was mixed with the Gag G122 peptide pool (P < 0.05). When used with DC (B), there were significant decreases in GL9, AL15, and GW15 peptide-specific IFN-γ production when the peptides were mixed with either the Gag 60/62 pool or the Gag G122 pool (P < 0.01).
Taken together, these results indicate that the lower T-cell responses to the larger pools of Gag peptides were related to lower T-cell viability and possibly competition among the peptides.
Enhanced T-cell proliferation stimulated by DC loaded with HIV-1 Gag and Nef peptides.
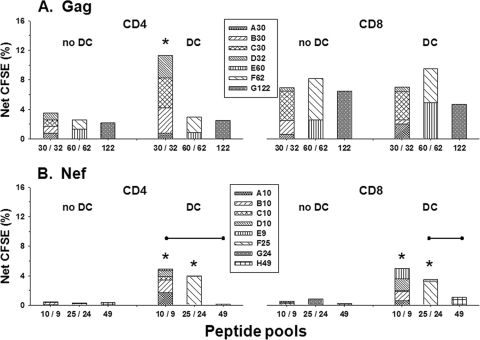
We next assessed peptide-specific, T-cell proliferation to DC loaded with viral peptides using a CFSE staining procedure (for a representative analysis, see Fig. S1 in the supplemental material). The initial results with peptide singlets indicated that DC from HIV-1-uninfected, EBV-seropositive persons that were loaded with EBV GL9, AL15, or GW15 peptide enhanced CD8+ T-cell proliferation compared to stimulation by the peptides without DC. Similar to IFN-γ production, greater T-cell proliferation was found for the N-terminal AL15 peptide than the C-terminal GW15 peptide. As expected with these MHC class I-restricted peptides, there was no stimulation of CD4+ T-cell proliferation (data not shown). We then assessed T-cell proliferation to the Gag and Nef peptide pools presented with or without DC from HIV-1-infected subjects on ART. The cumulative data show that there were lower T-cell responses to pools containing the larger numbers of Gag (Fig. 4A) and Nef (Fig. 4B) peptides. However, peptide-loaded DC induced significantly enhanced, CD4+ T-cell proliferative responses to the Gag 30/32 pools, and increases in proliferation of both CD4+ T and CD8+ T cells to the Nef 10/9 and 25/24 pools, compared to these peptide pools without DC (P < 0.05). Enhanced proliferative responses were also noted for CD4+ T cells in response to DC loaded with the Nef 10/9 pools compared to the Nef H49 pool (P < 0.05) and for CD8+ T-cell responses to Nef 25/24 pools compared to the Nef H49 pool (P < 0.05).
FIG. 4.
T-cell proliferation stimulated by pools of HIV-1 Gag and Nef peptides as determined by CFSE dye uptake. The different Gag (A) and Nef (B) peptide pools were compared for stimulation of T-cell proliferation with or without DC (n = 4 subjects; <50 copies/ml HIV-1 RNA). The bars represent the cumulative net percentages of T-cell proliferation for the peptide pools. DC enhanced the proliferative responses of CD4+ T cells to the Gag 30/32 pools and the responses of both CD4+ T and CD8+ T cells to the Nef 10/9 and Nef 25/24 pools, compared to stimulation with these peptides with no DC (*, P < 0.05). The data also show that with DC there were higher proliferative responses of CD4+ T cells to the Nef 10/9 pool than to the Nef H49 pool (•—•, P < 0.05) and higher proliferative responses of CD8+ T cells to the Nef 25/24 pool than to the Nef H49 pool (P < 0.05).
On day 6 when the CFSE assay was completed, the cell viability as detected by trypan blue dye uptake was lower in the cultures stimulated by the largest, Gag G122 peptide pool compared to the control cultures not stimulated with peptides, i.e., 40% ± 10% versus 66% ± 21%, respectively (n = 3; P < 0.05). There was also a trend of lower T-cell viability in cultures stimulated with the other peptide pools, i.e., 54% ± 7% for Gag 30/32, 46% ± 7% for Gag 60/62, 64% ± 7% for Nef 10/9, 61% ± 9% for Nef 25/24, and 53% ± 12% for Nef H49 (P = NS compared to the control). A decrease in cell viability was related to the lower T-cell proliferative response to the larger peptide pools as evidenced by a lower number of live cell events measured in the CFSE assay (data not shown).
Taken together, these results indicate that viral peptide-specific T-cell proliferation was enhanced by DC loaded with single peptides, or with pools of ≤30/32 Gag peptides and ≤10 Nef peptides. Without DC, the number of Gag and Nef peptides in a pool had minimal effects on T-cell proliferation, but with DC, the small pools (≤25 peptides) of HIV-1 Nef peptides stimulated greater T-cell proliferation than did the larger pools. The lower T-cell proliferation to the largest Gag G122 peptide pool was associated with lower cell viability.
Polyfunctional T-cell responses induced by DC loaded with Gag and Nef peptides.
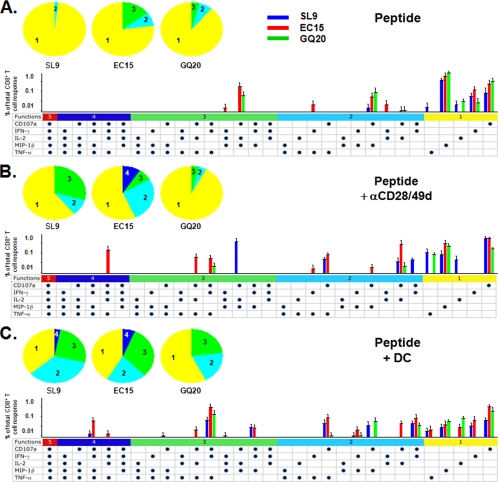
We next assessed the effects of DC on polyfunctional T-cell responses. For this, we compared stimulation of PBMC with peptide-loaded DC to peptide with and without the addition of αCD28/49d on production of T-cell immune mediators detected by ICS. DC from four HIV-1-infected subjects were loaded with the immunodominant, HLA A*0201-restricted, HIV-1 SL9 peptide and 15-mer and 20-mer extensions EC15 and GQ20 and used as APC in the ICS assay with autologous PBMC as responders. A representative analysis is shown in Fig. S2 in the supplemental material. Compared to the peptides alone (Fig. 5A) or peptides using αCD28/49d as a surrogate for APC (Fig. 5B), peptide-loaded DC (Fig. 5C) induced a greater magnitude of CD8+ T-cell polyfunctional responses (P < 0.01 and P < 0.05, respectively). Stimulation with peptides and αCD28/49d induced a level of ICS responses similar to that with the peptides alone (Fig. 5; P = NS).
FIG. 5.
Effect of DC on HIV-1 peptide-specific, polyfunctional immune mediator responses determined by ICS. PBMC from HLA A*0201, HIV-1-infected subjects on ART (n = 4 subjects; <50 copies/ml HIV-1 RNA) were stimulated with HLA A*0201-restricted Gag SL9, EC15, and GQ20 (5 μg/ml) without αCD28/49d or DC (A); with peptide with αCD28/49d (B); or with peptide with mature DC (C). The five-color pie charts show the relative proportions of immune mediators produced by the CD8+ T cells in groups of one to five per T cell, and the three-color bar graphs represent the actual percentages of T cells responding to SL9, EC15, and GQ20 peptides. The horizontal bars with different colors present the combinations of five, four, three, two, or one function. Each dot represents production of IL-2, IFN-γ, TNF-α, MIP-1β, or CD107a. Peptide-loaded DC induced greater CD8+ T-cell polyfunctional responses than did the peptides alone or peptides with αCD28/49d (P < 0.01 and P < 0.05, respectively). The magnitude of stimulation of T cells with peptides and αCD28/49d was not different from that for the peptides alone (P = NS).
The pattern of polyfunctional T-cell responses to these peptides differed among the three APC systems. Compared with peptide alone or the conventional ICS system with αCD28/49d, the SL9 peptide-loaded DC induced a higher number of polyfunctional T cells producing CD107a, IFN-γ, MIP-1β, and TNF-α (P < 0.05) (Fig. 5C). There was also a notable absence of IL-2 production by the CD8+ T cells in response to SL9-loaded DC (P < 0.05). The 15-mer peptide-loaded DC induced a higher number of polyfunctional T cells producing CD107a, IFN-γ, IL-2, MIP-1β, and TNF-α (P < 0.05). With αCD28/49d or the DC, the 20-mer peptide induced lower numbers of polyfunctional T cells than those induced by 9-mer or 15-mer peptides (P < 0.05). Thus, the conventional ICS system using αCD28/49d and the peptide revealed more polyfunctional T cells than did the peptides alone (pie charts in Fig. 5A and B). However, DC loaded with these peptides activated more polyfunctional T cells than did either peptide alone or peptide and αCD28/49d. We also observed a similar pattern of polyfunctional T-cell responses to EBV GL9 and its N-terminal flanking extension (AL15) and C-terminal flanking extension (GW15) in T cells from two HIV-1-uninfected subjects (data not shown).
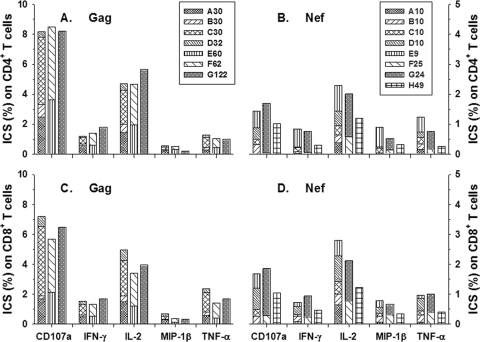
Due to the great complexity of using eight-color ICS to compare single Gag and Nef 15-mer peptides to the peptide pools for stimulation of T cells, we next determined ICS responses to different-sized HIV-1 peptide pools without DC. The cumulative data from five HIV-1-infected subjects on ART show that there were similar CD4+ and CD8+ T-cell reactivities to the different-sized Gag pools (Fig. 6A and C). Notably, there were greater numbers of CD4+ and CD8+ T cells producing CD107a than the four other immune mediators in response to the smaller Gag pools (A30, B30, C30, and D32) (P < 0.05) and IL-2 compared to IFN-γ, TNF-α, and MIP-1β (P < 0.05). Interestingly, the ICS responses to the larger pools of Gag peptides were similar to those to the smaller pools of Gag peptides, in contrast to the lower single-cell IFN-γ and proliferative T-cell responses to the larger Gag peptide pools. As expected, we found that the viability of cells in the unstimulated T-cell control (88% ± 6%) as detected by trypan blue dye uptake was lower in the ICS cultures stimulated with the pools containing the larger numbers of peptides (82% ± 12% for Gag 30/32, P = NS; 75% ± 11% for Gag 60/62, P < 0.05; 60% ± 13% for Gag G122, P < 0.01; 84% ± 13% for Nef 10/9, P = NS; 83% ± 11% for Nef 25/24, P = NS; 72% ± 13% for Nef H49, P < 0.05). However, we found that both CD4+ and CD8+ T cells from HIV-1-negative, uninfected controls produced several of these immune mediators in response to pools containing larger numbers of Gag peptides. That is, T cells from HIV-1-uninfected subjects had positive ICS responses to Gag 60/62 and Gag G122, which were TNF-α plus CD107a (three/three subjects), IL-2 alone (two/three subjects), IFN-γ alone (one/three subjects), and MIP-1β alone (one/three subjects), but not to the smaller pools of Gag peptides (data not shown). Similar results were seen in the cultures stimulated with pools containing a lower concentration of these peptides (2 μg/ml compared to 5 μg/ml; data not shown).
FIG. 6.
Cumulative immune mediator expression by CD8+ and CD4+ T cells to HIV-1 Gag and Nef peptide pools as determined by ICS. The bars represent cumulative data on percentages of IL-2, IFN-γ, TNF-α, MIP-1β, and CD107a produced by T cells from HIV-1-infected subjects on ART (n = 5 subjects; <50 copies/ml HIV-1 RNA) in response to the Gag and Nef peptide pools (5 μg/ml). The data show that there were no differences in production of the immune mediators by CD4+ and CD8+ T cells stimulated by the various Gag pools (A and C) and a trend of lower T-cell responses to the larger Nef peptide pools (B and D) (P = NS).
The CD4+ and CD8+ T-cell responses to Nef pools containing larger numbers of peptides, i.e., Nef H49, were lower in T cells stimulated with the larger Nef peptide pools (Fig. 6B and D) (P < 0.05). We did not find a nonspecific response of T cells from HIV-1-seronegative persons to the larger pools of Nef peptides, as we did for the Gag peptides (data not shown). There also were greater numbers of CD4+ T cells producing IL-2 compared to IFN-γ and MIP-1β after stimulation with the small Nef pools (A10, B10, C10, D10, and E9) (P < 0.05). The number of CD8+ T cells producing IL-2 and CD107a after stimulation by the small Nef pools was greater than the number of those producing IFN-γ, TNF-α, CD107a, or MIP-1β (P < 0.05). Finally, the number of CD8+ T cells producing CD107a was greater than the number of cells producing IFN-γ, TNF-α, CD107a, and MIP-1β as assessed by ICS (P < 0.05).
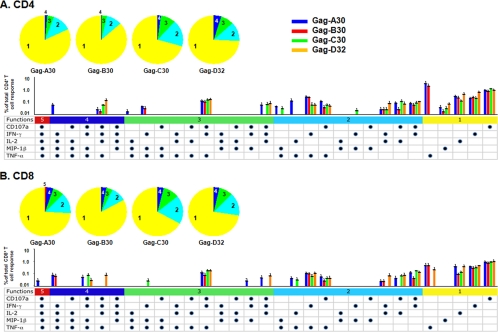
These results indicate that the optimal size of peptide pools for the most efficient stimulation of anti-HIV-1 CD8+ and CD4+ polyfunctional T cells was ≤30/32 Gag and ≤10 Nef peptides. Therefore, we next focused on whether DC loaded with the Gag 30/32 or Nef 10/9 pools stimulated production of multiple immune mediators by CD4+ and CD8+ T cells. We found that DC loaded with the four Gag 30/32 pools stimulated a broad array of polyfunctional CD4+ and CD8+ T-cell responses that did not differ in magnitude or pattern (Fig. 7A and B) (P = NS). The anti-Nef CD8+ T-cell polyfunctional responses were greater than the CD4+ T-cell responses for the combinations of four plus five, three plus four plus five, and two plus three plus four plus five immune mediators (Fig. 8A and B) (P = 0.038, 0.0003, and 0.00001, respectively). There were no differences in the patterns of immune mediators produced by either CD4+ or CD8+ T cells in response to the Nef peptides, except that there was no detectable production of MIP-1β by CD4+ T cells.
FIG. 7.
Stimulation of HIV-1 Gag-specific T-cell polyfunctional activity by DC loaded with Gag peptide pools. The five-color pie charts show the relative proportions of one to five immune mediators produced by the CD4+ (A) or CD8+ (B) T cells. The five-color bar graphs represent the percentages of T cells responding to the four different Gag 30/32 peptide pools, with different colors representing the combinations of five, four, three, two, or one function. Each dot represents production of IL-2, IFN-γ, TNF-α, MIP-1β, or CD107a. Cumulative immune mediator expression by the T cells is shown by pie charts, with bar graphs representing the mean % + standard error responses (n = 5 subjects; <50 copies/ml HIV-1 RNA) to the four Gag peptide pools.
FIG. 8.
Stimulation of HIV-1 Nef-specific T-cell polyfunctional activity by DC loaded with Nef peptide pools. The five-color pie charts show the relative proportions of one to five immune mediators produced by the CD4+ (A) or CD8+ (B) T cells. The five-color bar graphs represent the percentages of T cells responding to the five different Nef 10/9 peptide pools, with different colors representing the combinations of five, four, three, two, or one function. Each dot represents production of IL-2, IFN-γ, TNF-α, MIP-1β, or CD107a. Cumulative immune mediator expression by the T cells is shown by pie charts, with bar graphs representing the mean % + standard error responses (n = 5 subjects; <50 copies/ml HIV-1 RNA) to the five Nef peptide pools.
Taken together, these results indicate that DC from HIV-1-infected subjects enhance both monofunctional and polyfunctional CD8+ T-cell reactivity to single HIV-1 peptides representing the minimal, optimal HLA A*0201 epitope SL9, as well as to 15-mer and 20-mer extensions of this epitope. These CD8+ T-cell responses were greater than those induced in the conventional peptide-plus-αCD28/49d ICS assay. This enhancing effect of DC on CD8+ T-cell responses was similarly noted for EBV HLA A*0201-restricted peptides in EBV-seropositive, HIV-1-negative subjects. DC loaded with the smaller pools of Gag or Nef 15-mer peptides stimulated a broad array of monofunctional and polyfunctional CD8+ and CD4+ T-cell responses. As in the single-cell IFN-γ production and T-cell proliferation responses, there were lower numbers of CD8+ and CD4+ T cells producing immune mediators in response to larger pools of Nef peptides. Because dead cells were gated out in the ICS assay, this inhibitory effect was not likely to be related to loss of T-cell viability. However, ICS responses to the larger Gag peptide pools were associated with nonspecific T-cell reactivity in HIV-1-seronegative subjects.
DISCUSSION
HIV-1-specific T-cell responses are considered to be critical parameters of prevention of HIV-1 infection, host control of HIV-1 infection, and HIV-1 vaccine efficacy (10, 18, 39). Although processing and presentation of viral peptides by professional APC are required for optimal stimulation of CD4+ and CD8+ T-cell responses, most studies of anti-HIV-1 T-cell function have not considered the role of professional APC. We have previously reported that mature DC from HIV-1-infected subjects loaded with immunodominant, 9- to 10-mer HIV-1 peptides induce greater numbers of CD8+ T cells producing IFN-γ than does stimulation of the T cells with HIV-1 immunodominant peptides without DC or with peptide-loaded immature DC (20, 21). In the present study, we show that DC loaded with 9-mer viral epitopes or 15-mer, N- and C-terminal extensions of viral epitopes stimulated greater numbers of IFN-γ-producing T cells detected in the ELISPOT assay than did peptide-loaded DC. Interestingly, the greatest enhancement of T-cell reactivity was induced by DC that were loaded with the peptides after maturation with CD40L. Similarly, Dieckmann et al. (12) showed that DC matured with IL-1β, IL-6, prostaglandin E2, and TNF-α, but not immature DC, and then exogenously loaded with MHC class I-restricted peptides were superior in expanding CD8+ primary and recall T-cell responses to melanoma and influenza A virus immunodominant peptides, respectively. This contrasts with the prevailing view that mature DC are less capable than immature DC at processing of antigens for efficient MHC class I presentation (2). The mechanistic basis of our results needs to be defined, given that peptide loading into either immature or mature DC is relatively inefficient (54).
To address further their antigen-processing and antigen-presenting capacity and to reflect current conventions in the field, we loaded the DC with singlets and variously sized pools of 15-mer HIV-1 Gag and Nef peptides overlapping by 10 aa. We confirmed that mature DC loaded with singlets of 15-mers of Gag and Nef induced greater numbers of IFN-γ-producing T cells detected in the ELISPOT assay than did peptides without DC. More importantly, we extended this by showing that pools of 60 to 62 and 122 Gag peptides, and 24 to 25 and 49 Nef peptides, elicited lower T-cell reactivity than did pools of smaller numbers of peptides, i.e., pools of 30 to 32 Gag and 9 to 10 Nef peptides. This is similar to the findings of Russell et al. (45) that pools with lower numbers of overlapping 15-mers or 20-mers (2 to 10 HIV-1 peptides) induced more IFN-γ-producing T cells than did larger peptide pools (25 to 50 HIV-1 peptides). However, we extended this by showing a clear hierarchical increase in IFN-γ responses of the T cells to the smaller pools of Gag and Nef peptides, with the greatest cumulative responses being to the 15-mer singlets.
It is not clear why the large pools of Gag and Nef peptides induced lower T-cell reactivity than the smaller pools in the ELISPOT assay. Notably, we found that stimulation with the larger pools of HIV-1 peptides was associated with lower T-cell viability. This was not related to differences in amounts of the peptides, as we used the same final concentration of 5 μg/ml for each peptide in the pools. We extended this finding by showing that T cells from EBV-seropositive, HIV-1-seronegative, HLA A*0201 persons showed lower responses to DC loaded with the immunodominant EBV GL9 peptide or 15-mer N- or C-terminal extensions of GL9 if they were also loaded with the larger pools of HIV-1 Gag peptides, compared to DC loaded only with these EBV peptides. This competitive effect of the larger pools of Gag peptides was associated with lower T-cell viability. We conclude that, because these dying or dead cells were present in the ELISPOT assay, a toxic effect of the larger pools of peptides could have resulted in the lower numbers of IFN-γ-producing cells.
Many studies of HIV-1-specific T-cell immunity have used pools containing from 2 to over 100 HIV-1 synthetic peptides of 8 to 20 aa in length (3, 7, 9, 14, 17, 24, 25, 31, 32, 34, 37, 49). These peptides have been added directly to the PBMC or purified T cells to gauge peptide-specific immune responses in persons with HIV-1 infection or in anti-HIV-1 drug treatment and vaccine trials. Our results indicate, however, that stimulation of T cells with mature DC loaded with pools of ≤32 15-mer peptides of Gag and ≤10 15-mer peptides of Nef consistently yields greater IFN-γ responses in the ELISPOT assay. By such sensitive DC-based assays, we could detect low levels of antigen-specific T-cell responses that may otherwise be missed in such in vitro studies, clinical protocols, and vaccine trials of HIV-1 infection.
To define the broader capacity of DC to activate antigen-specific T cells, we assessed memory recall T-cell proliferation in response to DC loaded with 15-mers that require proteolytic processing and trafficking for efficient MHC class I presentation (43). Our results show that, as with IFN-γ production, DC from HIV-1-infected subjects on ART that were loaded with viral peptides could induce peptide-specific T-cell proliferation detected by CFSE staining that was superior to stimulation with the peptides alone. Similarly, there was a hierarchical proliferative response of CD8+ and CD4+ T cells dependent on the number of HIV-1 peptides in the pools, with lower T-cell reactivity to the larger pools of Gag and Nef peptides. Further examination of the T-cell proliferation responses revealed lower cell viability in the CFSE assays using the larger pools of HIV-1 peptides. Thus, cellular toxicity could at least in part be responsible for the lower T-cell proliferation induced by the larger peptide pools. Our results expand on the previous report of Newton et al. (35) that presentation of p24 recombinant antigen by DC partially restores impaired HIV-1-specific CD4+ T-cell proliferation associated with HIV-1 infection. This also indicates that studies of antigen-specific T-cell proliferation in HIV-1-infected subjects that do not include antigen-expressing, professional APC could be yielding suboptimal results.
Further evidence that DC are capable of enhancing multiple functions of antiviral T cells is provided by our results that mature DC loaded with HIV-1 peptides induced a broad, polyfunctional T-cell response. We found that production of IFN-γ, IL-2, TNF-α, MIP-1β, and CD107a in various combinations by CD8+ T cells was enhanced in response to DC loaded with the HIV-1 Gag epitope SL9 or its 15-mer and 20-mer extensions compared to these peptides without DC. This enhancing effect by DC on CD8+ T-cell responses was also observed for EBV HLA A*0201-restricted peptides in EBV-seropositive, HIV-1-negative subjects. Interestingly, CD8+ T cells did not produce IL-2 in response to SL9 presented by DC but did produce IL-2 in response to the peptide without DC. The basis of this difference in IL-2 response is unclear. It has been reported that CD8+ T cells from HIV-1-uninfected (23) or HIV-1-infected (50) persons that are stimulated for weeks in vitro with SL9 can produce IL-2. Interestingly, the magnitude of polyfunctional T-cell responses to these single SL9-based peptides was lower than some of the immune mediator levels reported in studies of HIV-1-infected progressors and nonprogressors (5). However, the HIV-1-infected study subjects used in these experiments were on long-term ART and had suppressed viral loads. This is known to result in lower T-cell reactivity as the HIV-1 antigen burden decreases, including polyfunctional immune mediator responses (16, 41).
We observed that the magnitude of the monofunctional and polyfunctional T-cell responses to DC loaded with SL9 or its EC15 and GQ20 extended peptides was greater than those induced by the peptides and αCD28/49d, which is a surrogate for APC used in most ICS assays (36, 52). This difference in T-cell activation is not unexpected, given that DC use other mechanisms for stimulating T cells besides triggering of CD28 and CD49 (2, 40). Indeed, triggering of CD28 via CD80 and CD86 could also activate regulatory CD4+ T cells that can inhibit T-cell immune effector responses (22).
To extend beyond polyfunctional T-cell responses to single HIV-1 peptides, we investigated the CD4+ and CD8+ T-cell reactivity by ICS to different-sized pools of Gag and Nef peptides without DC. We found that both CD4+ and CD8+ T cells produced multiple immune mediators in response to various-sized pools of Gag and Nef 15-mer peptides. Similar to our results with single-cell IFN-γ production and T-cell proliferation responses, there were greater numbers of CD8+ and CD4+ T cells producing immune mediators detected by ICS in response to the small pools of 9 to 10 Nef 15-mer peptides than to the larger, 24- to 25- and 49-Nef peptide pools. In contrast, there was no such decrease in ICS responses to the larger Gag peptide pools, even though there was lower cell viability in these cultures. We noted two possible bases for this result. First, dead cells were gated out in the ICS assay, thus proportionally increasing the percentage of ICS-positive T cells. Second, the larger Gag pools induced production of several of these immune mediators in T cells from HIV-1-seronegative persons. Thus, cell toxicity and nonspecific T-cell responses could have masked a lower, peptide-specific polyfunctional reactivity to the larger Gag pools and should be considered in ICS studies of T-cell immunity to Gag peptides in HIV-1-infected persons.
Importantly, our results show that DC loaded with each of the four pools of 30 to 32 Gag 15-mers stimulated a broad array of monofunctional and polyfunctional CD8+ and CD4+ T-cell responses that did not significantly differ in magnitude or pattern. There was also a polyfunctional response by CD8+ T cells to DC loaded with each of the five small pools of 9 to 10 Nef 15-mers, with various combinations of two, three, and four immune mediators. There was a less variable CD4+ T-cell response to these Nef peptides, with production of MIP-1β not being detected by ICS. This was also shown by a greater magnitude of CD8+ T-cell polyfunctional reactivity than the CD4+ T-cell responses for various combinations of the five immune mediators.
This study shows that DC can act as potent APC for activation of multiple, HIV-1 antigen-specific, T-cell functions. HIV-1 antigen-specific proliferation and production of multiple immune mediators by CD8+ and CD4+ T cells induced by DC could be central to control of HIV-1 infection. Although studies using conventional antigen stimulation methods without DC show that HIV-1-specific T-cell functions partially recover during ART (6, 28, 30, 38, 41, 47), our results indicate that use of autologous, mature DC as APC reveals greater antigen-specific, CD8+ and CD4+ T-cell reactivity. This enhancing effect on multiple T-cell functions could also occur in vivo in HIV-1-infected persons receiving preconditioned DC as immunotherapy or by targeting of resident DC for anti-HIV-1 T-cell sensitization (42).
Supplementary Material
Acknowledgments
We thank W. Jiang, P. Zhang, E. Molina, and K. Stojka for technical assistance; Mario Roederer (VRC/NIAID/NIH) for SPICE (version 4.1.6); W. Buchanan for clinical assistance; and the volunteers of the Pitt Men's Study (the Pittsburgh site of the Multicenter AIDS Cohort Study).
This work was supported by the National Institute of Allergy and Infectious Diseases P01 AI-055794, U01 AI-35041, and R37 AI-41870.
Footnotes
Published ahead of print on 19 August 2009.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Addo, M. M., R. Draenert, A. Rathod, C. L. Verrill, B. T. Davis, R. T. Gandhi, G. K. Robbins, N. O. Basgoz, D. R. Stone, D. E. Cohen, M. N. Johnston, T. Flynn, A. G. Wurcel, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2007. Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PLoS One 2:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Beattie, T., R. Kaul, T. Rostron, T. Dong, P. Easterbrook, W. Jaoko, J. Kimani, F. Plummer, A. McMichael, and S. Rowland-Jones. 2004. Screening for HIV-specific T-cell responses using overlapping 15-mer peptide pools or optimized epitopes. AIDS 18:1595-1598. [DOI] [PubMed] [Google Scholar]
- 4.Benito, J. M., M. Lopez, and V. Soriano. 2004. The role of CD8+ T-cell response in HIV infection. AIDS Rev. 6:79-88. [PubMed] [Google Scholar]
- 5.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binley, J. M., D. S. Schiller, G. M. Ortiz, A. Hurley, D. F. Nixon, M. M. Markowitz, and J. P. Moore. 2000. The relationship between T cell proliferative responses and plasma viremia during treatment of human immunodeficiency virus type 1 infection with combination antiretroviral therapy. J. Infect. Dis. 181:1249-1263. [DOI] [PubMed] [Google Scholar]
- 7.Ching, N., O. O. Yang, J. G. Deville, K. Nielsen-Saines, B. J. Ank, M. S. Sim, and Y. J. Bryson. 2007. Pediatric HIV-1-specific cytotoxic T-lymphocyte responses suggesting ongoing viral replication despite combination antiretroviral therapy. Pediatr. Res. 61:692-697. [DOI] [PubMed] [Google Scholar]
- 8.Currier, J. R., E. G. Kuta, E. Turk, L. B. Earhart, L. Loomis-Price, S. Janetzki, G. Ferrari, D. L. Birx, and J. H. Cox. 2002. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 260:157-172. [DOI] [PubMed] [Google Scholar]
- 9.Currier, J. R., U. Visawapoka, S. Tovanabutra, C. J. Mason, D. L. Birx, F. E. McCutchan, and J. H. Cox. 2006. CTL epitope distribution patterns in the Gag and Nef proteins of HIV-1 from subtype A infected subjects in Kenya: use of multiple peptide sets increases the detectable breadth of the CTL response. BMC Immunol. 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks, S. G., and B. D. Walker. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406-416. [DOI] [PubMed] [Google Scholar]
- 11.De Rosa, S. C., F. X. Lu, J. Yu, S. P. Perfetto, J. Falloon, S. Moser, T. G. Evans, R. Koup, C. J. Miller, and M. Roederer. 2004. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 173:5372-5380. [DOI] [PubMed] [Google Scholar]
- 12.Dieckmann, D., E. S. Schultz, B. Ring, P. Chames, G. Held, H. R. Hoogenboom, and G. Schuler. 2005. Optimizing the exogenous antigen loading of monocyte-derived dendritic cells. Int. Immunol. 17:621-635. [DOI] [PubMed] [Google Scholar]
- 13.Draenert, R., M. Altfeld, C. Brander, N. Basgoz, C. Corcoran, A. G. Wurcel, D. R. Stone, S. A. Kalams, A. Trocha, M. M. Addo, P. J. Goulder, and B. D. Walker. 2003. Comparison of overlapping peptide sets for detection of antiviral CD8 and CD4 T cell responses. J. Immunol. Methods 275:19-29. [DOI] [PubMed] [Google Scholar]
- 14.Dubey, S., J. Clair, T. M. Fu, L. Guan, R. Long, R. Mogg, K. Anderson, K. B. Collins, C. Gaunt, V. R. Fernandez, L. Zhu, L. Kierstead, S. Thaler, S. B. Gupta, W. Straus, D. Mehrotra, T. W. Tobery, D. R. Casimiro, and J. W. Shiver. 2007. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J. Acquir. Immune Defic. Syndr. 45:20-27. [DOI] [PubMed] [Google Scholar]
- 15.Fan, Z., X. L. Huang, P. Kalinski, S. Young, and C. R. Rinaldo, Jr. 2007. Dendritic cell function during chronic hepatitis C virus and human immunodeficiency virus type 1 infection. Clin. Vaccine Immunol. 14:1127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferre, A. L., P. W. Hunt, J. W. Critchfield, D. H. Young, M. M. Morris, J. C. Garcia, R. B. Pollard, H. F. Yee, Jr., J. N. Martin, S. G. Deeks, and B. L. Shacklett. 2009. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 113:3978-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham, B. S., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, J. E. Martin, M. M. McCluskey, B. K. Chakrabarti, L. Lamoreaux, C. A. Andrews, P. L. Gomez, J. R. Mascola, and G. J. Nabel. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 194:1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harari, A., and G. Pantaleo. 2008. HIV-1-specific immune response. Adv. Pharmacol. 56:75-92. [DOI] [PubMed] [Google Scholar]
- 19.Huang, K. H., M. R. Loutfy, C. M. Tsoukas, and N. F. Bernard. 2008. Immune correlates of CD4 decline in HIV-infected patients experiencing virologic failure before undergoing treatment interruption. BMC Infect. Dis. 8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, X. L., Z. Fan, L. Borowski, and C. R. Rinaldo. 2008. Maturation of dendritic cells for enhanced activation of anti-HIV-1 CD8(+) T cell immunity. J. Leukoc. Biol. 83:1530-1540. [DOI] [PubMed] [Google Scholar]
- 21.Huang, X. L., Z. Fan, B. A. Colleton, R. Buchli, H. Li, W. H. Hildebrand, and C. R. Rinaldo, Jr. 2005. Processing and presentation of exogenous HLA class I peptides by dendritic cells from human immunodeficiency virus type 1-infected persons. J. Virol. 79:3052-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunig, T. 2007. Manipulation of regulatory T-cell number and function with CD28-specific monoclonal antibodies. Adv. Immunol. 95:111-148. [DOI] [PubMed] [Google Scholar]
- 23.Kan-Mitchell, J., B. Bisikirska, F. Wong-Staal, K. L. Schaubert, M. Bajcz, and M. Bereta. 2004. The HIV-1 HLA-A2-SLYNTVATL is a help-independent CTL epitope. J. Immunol. 172:5249-5261. [DOI] [PubMed] [Google Scholar]
- 24.Kapogiannis, B. G., S. L. Henderson, P. Nigam, S. Sharma, L. Chennareddi, J. G. Herndon, H. L. Robinson, and R. R. Amara. 2006. Defective IL-2 production by HIV-1-specific CD4 and CD8 T cells in an adolescent/young adult cohort. AIDS Res. Hum. Retrovir. 22:272-282. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson, A. C., J. N. Martin, S. R. Younger, B. M. Bredt, L. Epling, R. Ronquillo, A. Varma, S. G. Deeks, J. M. McCune, D. F. Nixon, and E. Sinclair. 2003. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J. Immunol. Methods 283:141-153. [DOI] [PubMed] [Google Scholar]
- 26.Kern, F., N. Faulhaber, C. Frommel, E. Khatamzas, S. Prosch, C. Schonemann, I. Kretzschmar, R. Volkmer-Engert, H. D. Volk, and P. Reinke. 2000. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur. J. Immunol. 30:1676-1682. [DOI] [PubMed] [Google Scholar]
- 27.Kiecker, F., M. Streitz, B. Ay, G. Cherepnev, H. D. Volk, R. Volkmer-Engert, and F. Kern. 2004. Analysis of antigen-specific T-cell responses with synthetic peptides—what kind of peptide for which purpose? Hum. Immunol. 65:523-536. [DOI] [PubMed] [Google Scholar]
- 28.Lacabaratz-Porret, C., J. P. Viard, C. Goujard, Y. Levy, A. Rodallec, C. Deveau, A. Venet, and M. Sinet. 2004. Presence of HIV-specific CD4+ T-cell responses in HIV-infected subjects with sustained virologic control after highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 36:594-599. [DOI] [PubMed] [Google Scholar]
- 29.Lamoreaux, L., M. Roederer, and R. Koup. 2006. Intracellular cytokine optimization and standard operating procedure. Nat. Protoc. 1:1507-1516. [DOI] [PubMed] [Google Scholar]
- 30.Lange, C. G., M. M. Lederman, J. S. Madero, K. Medvik, R. Asaad, C. Pacheko, C. Carranza, and H. Valdez. 2002. Impact of suppression of viral replication by highly active antiretroviral therapy on immune function and phenotype in chronic HIV-1 infection. J. Acquir. Immune. Defic. Syndr. 30:33-40. [DOI] [PubMed] [Google Scholar]
- 31.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 32.Meddows-Taylor, S., S. Shalekoff, L. Kuhn, G. E. Gray, and C. T. Tiemessen. 2007. Development of a whole blood intracellular cytokine staining assay for mapping CD4(+) and CD8(+) T-cell responses across the HIV-1 genome. J. Virol. Methods 144:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 34.Mwau, M., A. J. McMichael, and T. Hanke. 2002. Design and validation of an enzyme-linked immunospot assay for use in clinical trials of candidate HIV vaccines. AIDS Res. Hum. Retrovir. 18:611-618. [DOI] [PubMed] [Google Scholar]
- 35.Newton, P. J., I. V. Weller, I. G. Williams, R. F. Miller, A. Copas, R. S. Tedder, D. R. Katz, and B. M. Chain. 2006. Monocyte derived dendritic cells from HIV-1 infected individuals partially reconstitute CD4 T-cell responses. AIDS 20:171-180. [DOI] [PubMed] [Google Scholar]
- 36.Nomura, L. E., J. M. Walker, and H. T. Maecker. 2000. Optimization of whole blood antigen-specific cytokine assays for CD4(+) T cells. Cytometry 40:60-68. [DOI] [PubMed] [Google Scholar]
- 37.Owen, R. E., E. Sinclair, B. Emu, J. W. Heitman, D. F. Hirschkorn, C. L. Epling, Q. X. Tan, B. Custer, J. M. Harris, M. A. Jacobson, J. M. McCune, J. N. Martin, F. M. Hecht, S. G. Deeks, and P. J. Norris. 2007. Loss of T cell responses following long-term cryopreservation. J. Immunol. Methods 326:93-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer, B. E., E. Boritz, N. Blyveis, and C. C. Wilson. 2002. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4+ T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J. Virol. 76:5925-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piacentini, L., C. Fenizia, V. Naddeo, and M. Clerici. 2008. Not just sheer luck! Immune correlates of protection against HIV-1 infection. Vaccine 26:3002-3007. [DOI] [PubMed] [Google Scholar]
- 40.Piguet, V., and R. M. Steinman. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehr, M., J. Cahenzli, A. Haas, D. A. Price, E. Gostick, M. Huber, U. Karrer, and A. Oxenius. 2008. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J. Virol. 82:3391-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinaldo, C. R. 2009. Dendritic cell-based human immunodeficiency virus vaccine. J. Intern. Med. 265:138-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock, K. L., I. A. York, T. Saric, and A. L. Goldberg. 2002. Protein degradation and the generation of MHC class I-presented peptides. Adv. Immunol. 80:1-70. [DOI] [PubMed] [Google Scholar]
- 44.Roederer, M., J. M. Brenchley, M. R. Betts, and S. C. De Rosa. 2004. Flow cytometric analysis of vaccine responses: how many colors are enough? Clin. Immunol. 110:199-205. [DOI] [PubMed] [Google Scholar]
- 45.Russell, N. D., M. G. Hudgens, R. Ha, C. Havenar-Daughton, and M. J. McElrath. 2003. Moving to human immunodeficiency virus type 1 vaccine efficacy trials: defining T cell responses as potential correlates of immunity. J. Infect. Dis. 187:226-242. [DOI] [PubMed] [Google Scholar]
- 46.Sercarz, E. E., and E. Maverakis. 2003. MHC-guided processing: binding of large antigen fragments. Nat. Rev. Immunol. 3:621-629. [DOI] [PubMed] [Google Scholar]
- 47.Teixeira, L., H. Valdez, J. M. McCune, R. A. Koup, A. D. Badley, M. K. Hellerstein, L. A. Napolitano, D. C. Douek, G. Mbisa, S. Deeks, J. M. Harris, J. D. Barbour, B. H. Gross, I. R. Francis, R. Halvorsen, R. Asaad, and M. M. Lederman. 2001. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS 15:1749-1756. [DOI] [PubMed] [Google Scholar]
- 48.Tobery, T. W., and M. J. Caulfield. 2004. Identification of T-cell epitopes using ELISpot and peptide pool arrays. Methods Mol. Med. 94:121-132. [DOI] [PubMed] [Google Scholar]
- 49.Tobery, T. W., S. A. Dubey, K. Anderson, D. C. Freed, K. S. Cox, J. Lin, M. T. Prokop, K. J. Sykes, R. Mogg, D. V. Mehrotra, T. M. Fu, D. R. Casimiro, and J. W. Shiver. 2006. A comparison of standard immunogenicity assays for monitoring HIV type 1 gag-specific T cell responses in Ad5 HIV type 1 gag vaccinated human subjects. AIDS Res. Hum. Retrovir. 22:1081-1090. [DOI] [PubMed] [Google Scholar]
- 50.Varela-Rohena, A., P. E. Molloy, S. M. Dunn, Y. Li, M. M. Suhoski, R. G. Carroll, A. Milicic, T. Mahon, D. H. Sutton, B. Laugel, R. Moysey, B. J. Cameron, A. Vuidepot, M. A. Purbhoo, D. K. Cole, R. E. Phillips, C. H. June, B. K. Jakobsen, A. K. Sewell, and J. L. Riley. 2008. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat. Med. 14:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasan, S., S. J. Schlesinger, and G. Arrode. 2007. T cell immune responses to HIV-1. Front. Biosci. 12:2330-2343. [DOI] [PubMed] [Google Scholar]
- 52.Waldrop, S. L., K. A. Davis, V. C. Maino, and L. J. Picker. 1998. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284-5295. [PubMed] [Google Scholar]
- 53.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zehn, D., C. J. Cohen, Y. Reiter, and P. Walden. 2006. Efficiency of peptide presentation by dendritic cells compared with other cell types: implications for cross-priming. Int. Immunol. 18:1647-1654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.