FIG. 2.
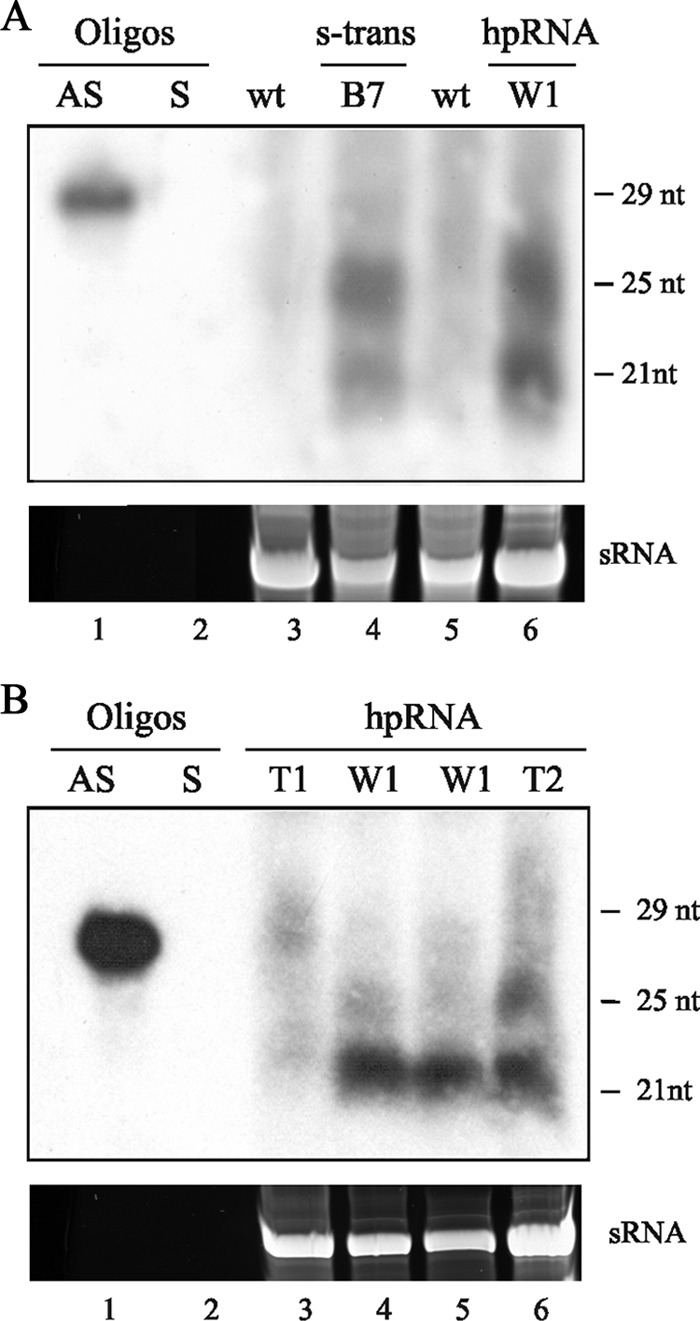
siRNA production in hpRNA-induced gene silencing. (A) Northern blot analysis of low-molecular-weight RNAs (50 μg) isolated from the wild-type strain R7B (lanes 3 and 5), a silenced albino transformant obtained by introduction of the sense transgene (s-trans) expressing plasmid pMAT647 into the wild-type strain (transformant B7; lane 4), and a silenced albino transformant obtained by introduction of the hpRNA-expressing plasmid pMAT1253 into the wild-type strain (transformant W1; lane 6). The cultures were grown for 24 h in liquid medium under continuous-illumination conditions. Oligos, oligonucleotides; AS, antisense; S, sense; wt, wild type. (B) Production of siRNAs in the dcl-2− genetic background. Northern blot analysis of low-molecular-weight RNAs (50 μg) isolated from cultures grown for 24 h in liquid medium from transformants of the dcl-2− mutant (transformant T1; lane 3) or the wild-type strain (transformant W1; lane 4) carrying the hpRNA-expressing transgene pMAT1253. Analysis of other dcl-2− transformants gave the same results. To detect siRNAs in the dcl-2− genetic background, cultures of one of the 15 dcl-2− transformants with a partially silenced phenotype (T2) and a wild-type silenced strain (W1) were grown for 48 h in solid minimal medium pH 3.2 (lanes 5 and 6). Ten picomoles per lane of 29-mer DNA oligonucleotide in antisense orientation (AS; lane 1) and 25-mer DNA oligonucleotide in sense orientation (S; lane 2) were used as size markers and to control the hybridization specificity. The RNA blot was hybridized with a hydrolyzed carB antisense-specific riboprobe, which corresponded to a 1,662-bp DNA fragment of the carB sequence that extends from position +863 to the end of the carB gene (18). The predominant RNA species in the small-RNA samples (sRNA) were stained with ethidium bromide after size separation by agarose gel electrophoresis.
