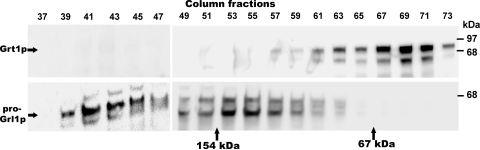
FIG. 1.
Grt1p and Grl1p are present in distinct transport complexes. A crude membrane fraction from SB281 cells was solubilized in Triton X-100, as described in Materials and Methods, and the cleared solute was applied to a Sephacryl S300 column in the same buffer. A void volume marker, blue dextran, eluted at fraction 39. Beginning with eluted fraction 37, the proteins in alternate fractions were resolved using SDS-PAGE, transferred to nitrocellulose, and Western blotted with polyclonal antibodies against Grt1p (upper panel) or Grl1p (lower panel) on parallel nitrocellulose filters. Fractions 37 to 47 were fitted on one blot, and fractions 49 to 73 were fitted on a separate blot and developed under identical conditions. Grl1p was found predominantly near the column void volume, consistent with formation of large heterooligomeric Grl-based complexes. Grt1p eluted in later column fractions, peaking near fraction 69. Two molecular mass markers were run in parallel, and their peak elution positions are shown. The bacteriophage N4 gp65 monomer (154 kDa) peaked in fraction 52, and BSA (67 kDa) peaked in fraction 66.