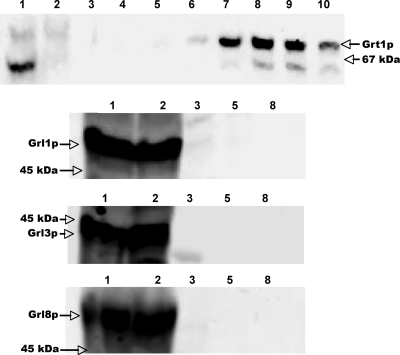
FIG. 2.
Grt1p does not copurify with Grl proteins. Grt1p-His6 was expressed in SB281 cells and purified from detergent lysates by nickel-affinity chromatography as described in Materials and Methods. (Top panel) Nickel column fractions were subjected to SDS-PAGE and analyzed by Western blotting using anti-Grt1p antibodies. The upper band corresponds to Grt1p; the lower band is a cross-reactive species seen in SB281 lysates. Lane 1, lysate applied to column. lane 2, column flowthrough; lanes 3 to 10, elution with increasing concentrations of imidazole, with each fraction representing 1 column volume (fractions 3 and 4, 40 mM; 5 to 7, 60 mM; 8 to 10, 250 mM). Grt1p-His6 elutes primarily in fractions 7 to 9. Arrows on the right indicate the band corresponding to Grt1p and the position of the 67-kDa SDS-PAGE standard, BSA. The remaining three panels show the identical column fractions analyzed by Western blotting with antibodies against Grl1p, Grl3p, and Grl8p. Column fractions correspond to those in the top panel. In all cases, the Grl proteins are found in the column flowthrough and do not copurify with Grt1p-His6.