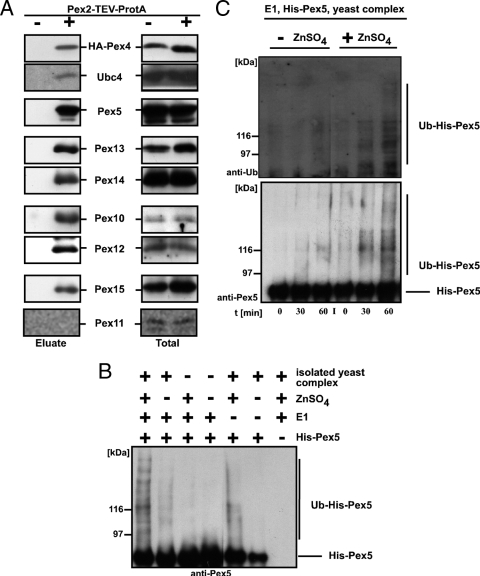
FIG. 2.
The RING complex exhibits Ub ligase activity. (A) The membrane-bound protein import complex was isolated from digitonin-solubilized membranes of wild-type cells expressing Pex2-TEV-ProtA as well as HA-tagged Pex4 via IgG-Sepharose and subsequent TEV protease cleavage. As a control, wild-type cells expressing no ProtA fusion protein were treated equally. The corresponding digitonin-solubilized membranes (totals) are shown in the right column. Indicated proteins of the TEV protease eluates and totals were detected by immunoblot analysis. (B and C) Recombinant Pex5 was incubated in the presence or absence of recombinant E1, zinc ions, and the Pex2 complex isolated from pex5Δ membranes. Reactions were stopped by the addition of SDS sample buffer after 45 min (B) or indicated time points (C), and modified Pex5 species were detected by immunoblot analysis. Ubiquitinated Pex5 was clearly detectable only when all constituents were present. Lack of either zinc or E1 led to significantly smaller amounts of modified Pex5.