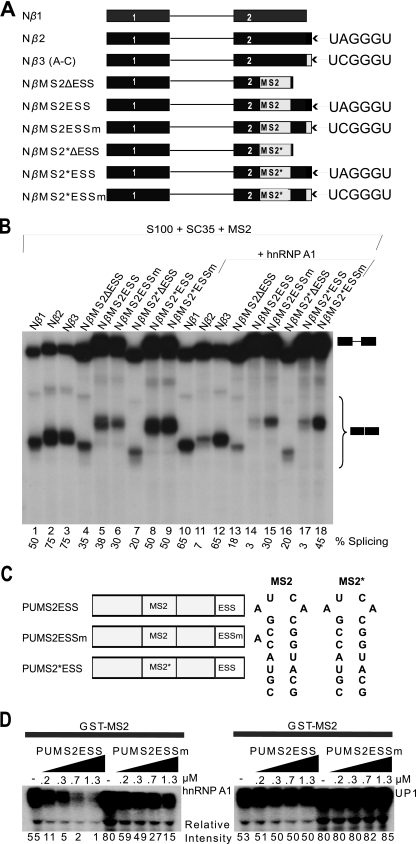
FIG. 3.
Unwinding of RNA secondary structure and splicing inhibition by hnRNP A1. (A) β-Globin minigenes with or without MS2 or MS2 mutant (MS2*) hairpins. (B) In vitro splicing of capped Nβ-globin minigene transcripts (1.15 nM) in S100 extract complemented with 0.4 μM SC35, in the presence of 1.73 μM GST-MS2 coat protein, and with or without 0.6 μM hnRNP A1. (C) Poly(U) MS2 constructs to test the unwinding activity of hnRNP A1 as it binds cooperatively. The sequences and secondary structures of MS2 and MS2* are shown next to the construct diagrams. (D) GST-MS2 pulldowns. Labeled RNA (15 nM) was first incubated with GST-MS2 protein (1.5 μM), followed by incubation with increasing concentrations of recombinant hnRNP A1 (left panel; 0 to 1.3 μM) or UP1 (right panel; 0 to 1.3 μM) and then incubation with GST-agarose beads. After washing, bound RNA was eluted, separated by denaturing PAGE, and detected by autoradiography. Band intensities were measured on a phosphorimager, and normalized values relative to the lowest band intensity are shown below the gels.