Abstract
Controversy remains regarding the mechanisms of acupuncture analgesia. A prevailing theory, largely unproven in humans, is that it involves the activation of endogenous opioid antinociceptive systems and μ-opioid receptors (MORs). This is also a neurotransmitter system that mediates the effects of placebo-induced analgesia. This overlap in potential mechanisms may explain the lack of differentiation between traditional acupuncture and either non-traditional or sham acupuncture in multiple controlled clinical trials. We compared both short- and long-term effects of traditional Chinese acupuncture (TA) versus sham acupuncture (SA) treatment on in vivo MOR binding availability in chronic pain patients diagnosed with fibromyalgia (FM). Patients were randomized to receive either TA or SA treatment over the course of four weeks. Positron emission tomography (PET) with 11C-carfentanil was performed once during the first treatment session and then repeated a month later following the eighth treatment. Acupuncture therapy evoked short-term increases in MOR binding potential, in multiple pain and sensory processing regions including the cingulate (dorsal and subgenual), insula, caudate, thalamus, and amygdala. Acupuncture therapy also evoked long-term increases in MOR binding potential in some of the same structures including the cingulate (dorsal and perigenual), caudate, and amygdala. These short- and long-term effects were absent in the sham group where small reductions were observed, an effect more consistent with previous placebo PET studies. Long-term increases in MOR BP following TA were also associated with greater reductions in clinical pain. These findings suggest that divergent MOR processes may mediate clinically relevant analgesic effects for acupuncture and sham acupuncture.
Keywords: acupuncture, opioid, mu, fibromyalgia, pain, positron emission tomography
Introduction
Acupuncture as a component of East-Asian medical systems has been used to treat pain for over two millennia however the cellular and molecular constituents of this therapy remain largely unknown. Prevailing theories, arising largely from studies in animals, suggest that endogenous opioids and their associated receptors are involved in this treatment (He et al., 1985; Pert et al., 1981; Ho and Wen, 1989; Pomeranz and Chiu, 1976; Chen et al., 1996). Most studies have focused on the opioid neurotransmitters (Stux and Hammerschlag R, 2001), where enhanced release seems to accompany needle insertion, however less attention has been paid to the opioid receptors themselves (e.g. the μ, κ, and δ opioid receptor classes) and their relationship with clinical response.
Recent controversy in the field of acupuncture research was generated when several large scale randomized controlled trials in chronic pain patients failed to show superiority of acupuncture over sham acupuncture methods (Brinkhaus et al., 2006; Linde et al., 2005; Melchart et al., 2005; Harris et al., 2005). This has lead opponents of acupuncture therapy to suggest that it is no more effective than a placebo intervention. Since placebo administration also induces activation of opioid receptors, specifically the μ-opioid receptor (MOR) class (Benedetti and Amanzio, 1997; Zubieta et al., 2005; Amanzio and Benedetti, 1999; Levine et al., 1978; Pomeranz and Chiu, 1976), acupuncture may indeed operate in part via placebo mechanisms.
Neuroimaging methods allow for the ability to explore the central neurobiological mechanisms of both acupuncture and placebo interventions. Recent functional magnetic resonance imaging (fMRI) studies demonstrate deactivation of limbic structures including the amygdala, the hippocampus, and the perigenual cingulate via a mechanism that is distinct from pain and sham stimulation (Hui et al., 2000; Hui et al., 2005; Napadow et al., 2007). Thus while traditional acupuncture and sham acupuncture may have equivalent analgesic effects they may differ significantly in their underlying neurobiological response.
Here we directly explore the involvement of the endogenous opioid system during acupuncture treatment of chronic pain patients diagnosed with fibromyalgia (FM) (Wolfe et al., 1995). FM is a relatively common chronic pain condition thought to originate from augmented pain processing in the central nervous system (Gracely et al., 2002). We have previously demonstrated that FM patients have reduced central μ-opioid receptor (MOR) binding potential (BP; an in vivo measure of binding availability) using 11C-carfentanil (CFN) positron emission tomography (PET) with the μ-opioid selective radiotracer [11C]carfentanil (Harris et al., 2007). In that study patients with greater clinical pain displayed reduced MOR BP. Here we perform CFN PET on FM patients before and following acupuncture and sham treatment. We reasoned that dynamics in receptor binding could complement previous acupuncture research which has focused largely on the release of endogenous opioids. One study has examined acupuncture effects on central opioid receptor binding, however that study used a non-selective opioid receptor agonist and did not examine effects within a clinical population (Dougherty et al., 2008).
Based on animal data and in vitro measures of MOR binding (Gao et al., 1997), it was hypothesized that long-term acupuncture therapy may result in increased MOR BP, or receptor availability in vivo. Further, we reasoned that these effects would not be observed in the sham treatment group, thus differentiating “placebo” from active treatment conditions. Finally, since regional decreases in MOR BP have been associated with greater clinical pain in FM patients (Harris et al., 2007), increases in BP were expected to be associated with reduced clinical pain.
Materials and Methods
Participants
As part of a study investigating the impact of acupuncture treatment in FM, 20 female patients (mean(SD) age:44.3(13.6) yrs) were examined with two CFN PET imaging sessions. Participants were randomized to receive either nine traditional acupuncture (TA; n=10) or nine non-skin penetrating sham acupuncture (SA; n=10) treatments. Demographics of the sample population are presented in Supplementary Table 1. No significant differences were detected between participants in the TA and SA groups for either age, race, duration of FM symptoms, or pre-treatment clinical pain scores. Participants gave written informed consent and all study protocols were approved by the University of Michigan Institutional Review Board and the Radioactive Drug Research Committee.
All participants: 1) met the American College of Rheumatology (1990) criteria (Wolfe et al., 1990) for the diagnosis of FM for at least 1 year; 2) had continued presence of pain more than 50% of days; 3) were willing to limit the introduction of any new medications or treatment modalities for control of FM symptoms during the study; 4) were over 18 and under 75 years of age; 5) were female; 6) were right handed; 7) had no alcohol intake 48 hours prior to PET studies; and 8) were capable giving written informed consent. Participants were excluded if they: 1) had previous experience with acupuncture; 2) had current use or a history of use of opioid or narcotic analgesics; 3) had a history of substance abuse; 4) had the presence of a known coagulation abnormality, thrombocytopenia, or bleeding diathesis that may preclude the safe use of acupuncture; 5) had the presence of concurrent autoimmune or inflammatory disease such as rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, etc. that causes pain; 6) had concurrent participation in other therapeutic trials; 7) were pregnant and nursing mothers; 8) had severe psychiatric illnesses (current schizophrenia, major depression with suicidal ideation, substance abuse within two years); 9) had current major depression; or 10) had contraindications to PET. Concomitant medications are listed in Supplementary Table 2.
Positron Emission Tomography (PET)
Image Acquisition
Scans were acquired with a Siemens (Knoxville, TN) HR+ scanner in 3-D mode (reconstructed FWHM resolution ~5.5 mm in-plane and 5.0 mm axially), with septa retracted and scatter correction. Participants were positioned in the PET scanner gantry, and an intravenous (antecubital) line was placed in the right arm. A light forehead restraint was used to eliminate intrascan head movement. CFN was synthesized at high specific activity (> 2000 Ci/mmol), as previously described (Jewett, 2001); 10–15 mCi (370–555 MBq) were administered during the scan. Fifty percent of the CFN dose was administered as a bolus, and the remaining 50% by continuous infusion for the remainder of the study. Twenty-eight frames of images were acquired over 90 minutes with an increasing duration (30 sec up to 10 min). The total mass of carfentanil administered was maintained below 0.03 μg/kg, ensuring that the compound was administered in a tracer quantity (i.e. a sub-pharmacological dose). Receptor occupancy by this mass of carfentanil is estimated to be 0.2% to 0.6% depending on the brain region (Gross-Isseroff et al., 1990; Gabilondo et al., 1995). The methodology employed to quantify MOR sites (i.e., bolus-continuous infusion to more rapidly achieve full equilibrium conditions across kinetic compartments and see Logan plot models below) has been shown not to be significantly susceptible to changes in blood flow, and therefore tracer transport, that could be caused by procedures such as acupuncture (Zubieta et al., 2003b; Joshi et al., 2008).
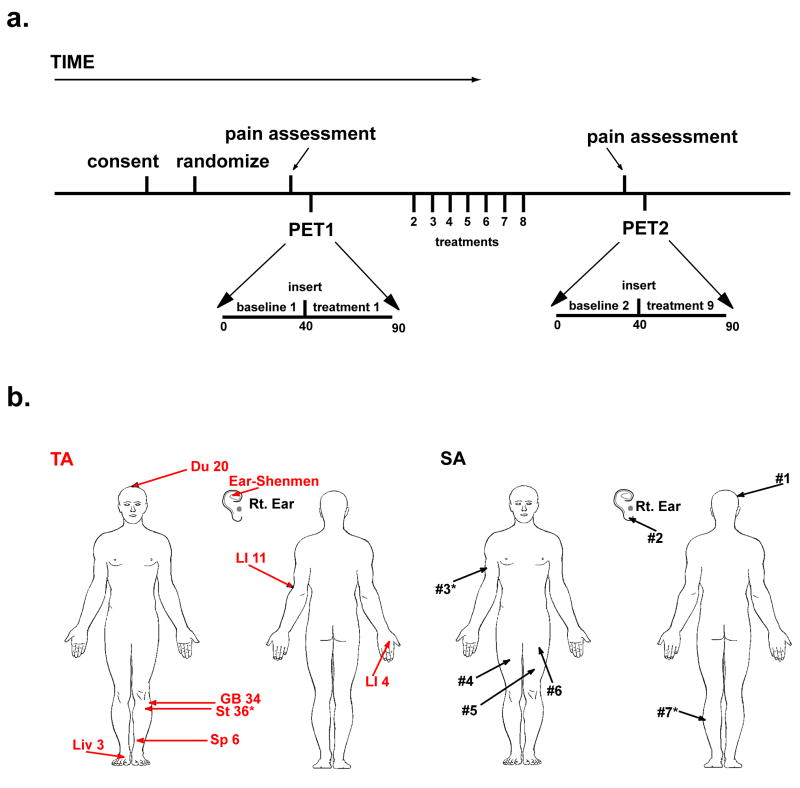
Figure 1A displays the timeline for PET image acquisition, treatment procedures, and study outcomes. For the first and second PET image session, PET1 and PET2 respectively, we used data from minutes 10 to 40 as our baseline measurement because the slope of the Logan plot (see below) for CFN becomes linear at approximately 7.5 minutes following CFN infusion (Zubieta et al., 2003b). This was followed by TA and SA procedures (see below) performed between minutes 40 to 45. Data from minutes 40 to 45 were omitted due to head motion during treatment procedures. After needle insertion and manipulation, scans from 45 to 90 minutes during PET1 were used as the short-term treatment measurement (i.e. treatment1). During minutes 45 to 90, needles were retained in the TA group, whereas no needles were present in the SA group since SA did not involve skin penetration. For analysis of long-term changes in MOR binding, changes between PET1 and PET2 baseline scans, baseline1 and baseline2 respectively, were examined.
Figure 1.
Study Design. A) Participant timeline from consent, through PET imaging sessions, and treatments. Following consent, all participants were randomized to either the traditional acupuncture (TA) or sham acupuncture (SA) treatment groups. Immediately before both PET imaging sessions (i.e. PET1 and PET2), participants completed the SF MPQ to assess clinical pain. During PET1, participants underwent a baseline scan (baseline1) and a treatment scan (treatment1) both of which were used to estimate short-term effects on MOR binding. Participants then received seven acupuncture or sham treatments outside of the scanner. This was followed by PET2, a second imaging session. The baseline scan during PET2 was used for comparison with the baseline scan in PET1 to estimate long-term changes in resting MOR binding. B) TA (red) and SA (black) point locations. Similar body regions were used for both interventions.
Anatomical MRI scans were acquired in all subjects on a 3 Tesla scanner (Signa LX, General Electric, Milwaukee, WI). The acquisition sequence was axial SPGR Inverse Recovery-Prepared MR [echo time (TE) = 3.4 ms, repetition time (TR) = 10.5 ms, inversion time (TI) = 200 ms, flip angle = 25°, number of excitations (NEX) = 1, using 124 contiguous images, 1.5 mm-thickness).
Image Processing
Images were reconstructed using iterative algorithms (brain mode; FORE/OSEM 4 iterations, 16 subsets; no smoothing) into a 128 × 128 pixel matrix in a 28.8 cm diameter field of view. Attenuation correction was performed through a 6-min transmission scan (68Ge source) obtained prior to each PET study, also with iterative reconstruction of the blank/transmission data followed by segmentation of the attenuation image. Small head motions during emission scans were corrected by an automated computer algorithm for each subject before analysis, and the images were co-registered to each other with the same software (Minoshima et al., 1993). Time points were then decay-corrected during reconstruction of the PET data. Image data were then transformed on a voxel-by-voxel basis into two sets of parametric maps: (a) a tracer transport measure (K1 ratio), and (b) a receptor-related measure (DVR). To avoid the need for arterial blood sampling, the tracer transport and binding measures were calculated using a modified Logan graphical analysis (Logan et al., 1996), using the occipital cortex (an area devoid of MORs) as the reference region. The slope of the Logan plot was used for the estimation of the distribution volume ratio (DVR), a measure equal to the f2(Bmax/Kd) + 1 for this receptor site and radiotracer. Bmax/Kd (or DVR-1) is the receptor related measure or binding potential (BP). The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value. K1 and DVR images for each experimental period and MR images were co-registered to each other and to the International Consortium for Brain Mapping (ICBM) stereotactic atlas orientation. The accuracy of coregistration and non-linear warping algorithms was confirmed for each subject individually by comparing the transformed MRI and PET images to each other and the ICBM atlas template.
Group differences were mapped into stereotactic space using t maps of statistical significance with SPM99 (Wellcome Department of Cognitive Neurology, London, UK) and Matlabv6.5 (MathWorks, Natick MA) software, with a general linear model. No global normalization was applied to the data, and therefore the calculations presented are based on absolute Bmax/Kd estimates. Only regions with specific MOR BP were included in the analyses (i.e. voxels with DVR values>1.1, or BP >0.1). To compensate for small residual anatomic variations across subjects and to improve signal to noise ratios, a three-dimensional Gaussian filter (FWHM 6 mm) was applied to each scan.
Image Analysis
Short-term changes in MOR BP were detected using two sample t-tests between patients receiving TA and SA between experimental conditions in PET1 on a voxel-by-voxel basis using SPM99: (Comparison I = [TA(treatment1-baseline1) > SA(treatment1-baseline1)] and Comparison II = [SA(treatment1-baseline1) > TA(treatment1-baseline1)]). Significant effects were detected for each comparison using two separate approaches: 1) an entire image-wide search that was unconstrained by regional predictions and 2) a regional approach that was based on a priori hypotheses. For the latter approach, a priori regions that had either been previously identified as involved in MOR mediated antinociception in humans (Zubieta et al., 2001; Zubieta et al., 2005) or PET trials using acupuncture (Biella et al., 2001) were determined using a standard brain atlas. These regions included: cingulate cortex, insula, nucleus accumbens, caudate, putamen, thalamus, hypothalamus, amygdala, and periaqueductal grey. When effects of treatment were observed in these regions we employed an uncorrected statistical threshold of p<0.001 with a minimum cluster size of 20 voxels. These typically had z-scores between 3.3 and 4.3 for this analysis. For brain regions not previously hypothesized, significant regions were identified with a threshold of p<0.05 after correction by multiple comparisons using family wise error approach (Friston et al., 1995a; Friston et al., 1995b). These typically had z-scores > 4.3 for this analysis.
Long-term changes in MOR BP were also detected using two sample t-tests between patients receiving TA and SA between baseline scans in PET1 versus PET2 on a voxel-by-voxel basis using SPM99: (Comparison I = [TA(baseline2-baseline1) > SA(baseline2-baseline1)] and Comparison II = [SA(baseline2-baseline1) > TA(baseline2-baseline1)]). Identical to the short-term changes, significant effects were detected for each comparison using two separate approaches: 1) an entire image-wide search that was unconstrained by regional predictions and 2) a regional approach that was based on a priori hypotheses. When effects of treatment were observed in a priori regions we employed an uncorrected statistical threshold of p<0.001 with a minimum cluster size of 20 voxels. These typically had z-scores between 3.1 and 4.5 for this analysis. For brain regions not previously hypothesized, significant regions were identified with a threshold of p<0.05 after correction by multiple comparisons using the family wise error approach (Friston et al., 1995a; Friston et al., 1995b). These typically had z-scores > 4.5 for this analysis.
Positive and negative correlations between long-term changes in MOR BP (baseline2 – baseline1) and changes in clinical pain (post – pre treatment) were performed using SPM99 again using a voxel-wise whole brain approach and an a priori region approach. Only regions showing significance after correction for multiple comparisons (i.e. p<0.05 corrected) are reported.
Numerical values for MOR binding were extracted from the image data by averaging the values of voxels contained in an area where significant effects were obtained in the voxel-by-voxel analyses, down to a threshold of p = 0.01. These values were then entered into SPSS version 14.0 (Chicago, IL) for plotting and assessment of possible outliers.
Treatment
We used an acupuncture treatment protocol previously utilized in a large clinical trial of acupuncture versus sham acupuncture in FM patients (Harris et al., 2005). This protocol was used because: 1) participants could not determine whether they receiving real or sham acupuncture, and 2) this led to robust effects on chronic pain in both groups. Thus, this seemed an ideal protocol to isolate differences in mechanisms between acupuncture and sham acupuncture, in a group of chronic pain patients. During TA 9 acupuncture needles were inserted: Du20, ear Shenmen, LI4, LI11, Sp6, Liv3, GB34 and bilateral St36 (Figure 1B). All needles below the neck level were manually manipulated to elicit De Qi sensations. SA participants received a non-skin penetrating pricking sensation at 9 non-acupuncture point locations using a previously validated sham procedure (Sherman et al., 2002). The sham locations were within similar body locations as the TA points however they were not on known acupuncture points or meridians. The length of time was similar for needle insertion and manipulation for TA and skin pricking for SA. All participants were blindfolded during each treatment to prevent patient knowledge of treatment assignment.
Clinical Pain
Clinical pain was assessed immediately prior to PET1 and PET2 with the Short Form of the McGill Pain Questionnaire (SF MPQ) (Melzack, 1987). The SF MPQ has two subscales that measure “sensory” and “affective” qualities of pain.
Assessment of Masking
Following the first PET session, participants were asked to guess which treatment they thought they had been assigned to. The three choices were: 1) “Acupuncture”, 2) “Sham Acupuncture”, and 3) “Don’t know”. A Chi-squared test was used to determine whether there was a significant difference between groups (i.e. unmasking or unblinding of the trial).
Results
Short-Term Differential Changes in MOR BP During Acupuncture and Sham Treatment
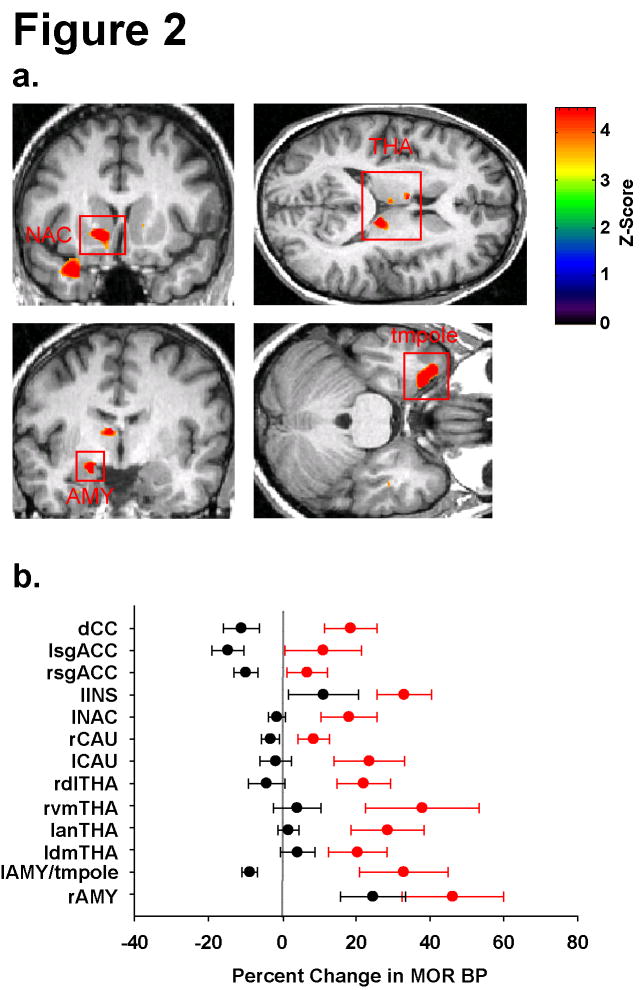
The design of this trial is depicted in Figure 1. Short-term differences in MOR BP between TA and SA treatments were examined with two separate comparisons: Comparison I = [TA(treatment1-baseline1) > SA(treatment1-baseline1)] and Comparison II = [SA(treatment1-baseline1) > TA(treatment1-baseline1)]. 14 regions were identified as having differences in MOR BP between groups with Comparison I (Table 1 and Figure 2). No regions were detected with Comparison II that met significance after correction for multiple comparisons (see Supplementary Figure 1A glass brain results). Inspection of Table 1 and Figure 2 indicates that treatment differences were attributable largely to increases in MOR BP following TA whereas SA evoked either a small decrease in MOR BP or resulted in no change. Two exceptions were the right amygdala and left insula which showed increases in BP for both groups, albeit larger increases for traditional acupuncture. Within the regions identified, the cingulate cortex, the nucleus accumbens, the thalamic nuclei, amygdala, and the temporal pole form part of an endogenous opioid circuit known to participate in the regulation of sensory and affective qualities of pain, as well as in emotional responses in humans (Zubieta et al., 2003a; Zubieta et al., 2001; Kennedy et al., 2006; Zubieta et al., 2003b).
Table 1.
Regions Displaying Short-Term Increases in MOR Binding Following Acupuncture
| Region | MNI Coordinates | Voxels | Z | Acupuncture (TA) BP mean(s.e.m.) | Sham (SA) BP mean(s.e.m.) | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Pre | Post | Pre | Post | |||
| Glbl V | 0.62(0.03) | 0.61(0.03) | 0.58(0.03) | 0.55(0.03) | |||||
| dCC | −1 | 1 | 34 | 178 | 4.2 | 0.83(0.10) | 0.95(0.09) | 1.01(0.12) | 0.87(0.08) |
| lsgACC | −11 | 20 | −20 | 120 | 4.1 | 1.09(0.09) | 1.18(0.11) | 1.06(0.09) | 0.90(0.09) |
| rsgACC | 2 | 35 | −20 | 399 | 3.9 | 1.42(0.07) | 1.51(0.10) | 1.39(0.12) | 1.24(0.10) |
| lINS | −42 | 4 | −20 | 540 | 3.6 | 0.91(0.06) | 1.22(0.12) | 0.92(0.07) | 1.00(0.08) |
| lNAC | −12 | 12 | −6 | 1004 | 7.2** | 2.38(0.12) | 2.81(0.23) | 2.26(0.11) | 2.22(0.11) |
| rCAU | 14 | 16 | 2 | 212 | 3.9 | 2.12(0.10) | 2.30(0.14) | 1.87(0.10) | 1.81(0.10) |
| lCAU | −9 | 7 | 6 | 140 | 4.1 | 1.02(0.13) | 1.23(1.15) | 0.98(0.09) | 0.94(0.07) |
| dlTHA | 11 | −26 | 11 | 547 | 5.9** | 1.41(0.14) | 1.73(0.21) | 1.36(0.09) | 1.29(0.10) |
| vmTHA | 2 | −9 | −5 | 91 | 4.9* | 0.95(0.09) | 1.29(0.18) | 1.00(0.12) | 1.03(0.11) |
| aTHA | −7 | −4 | 10 | 203 | 4.4* | 1.17(0.11) | 1.46(0.14) | 1.21(0.10) | 1.21(0.08) |
| dmTHA | −5 | −17 | 12 | 54 | 4.1 | 1.80(0.19) | 2.08(0.21) | 1.71(0.15) | 1.75(0.13) |
| lAMY/tmpole | −26 | 11 | −30 | 1556 | 7.1** | 1.06(0.08) | 1.39(0.14) | 1.21(0.09) | 1.10(0.08) |
| rAMY | 18 | −4 | −25 | 34 | 3.3 | 0.79(0.07) | 1.17(0.16) | 0.88(0.11) | 1.06(0.12) |
p<0.001 corrected;
p<0.05 corrected; all other regions p<0.001 uncorrected
Region Definitions: (Glbl V) global value; (CC) cingulate cortex; (ACC) anterior cingulate cortex; (INS) insula; (NAC) nucleus accumbens; (CAU) caudate; (THA) thalamus; (AMY) amygdala; (tmpole) temporal pole; (l: left; r: right; a: anterior; d: dorsal; dl: dorsal lateral; dm: dorsal medial; sg: subgenual; vm: ventral medial)
Figure 2.
Differential Short-Term Effects of Acupuncture and Sham Acupuncture on MOR Binding. A) Regions of interest showing increased MOR BP following acupuncture as compared to sham treatment. upper left: left nucleus accumbens (lNAC), upper right: three thalamic regions (THA), lower left and right: left amygdala (lAMY), and temporal pole (ltmpole) respectively. B) Percent changes and S.E.M. in MOR BP (treatment1 – baseline1) for all regions identified. Red circles (TA) and black circles (SA) represent group mean values with standard error bars. Overall acupuncture resulted in increases in MOR BP with sham treatment resulting largely in either no change or small decreases in BP.
To investigate whether the observed differences between TA and SA could be due to baseline differences between treatment groups, we compared baseline MOR BP values for the above 14 regions. None of the regions of interest (ROIs) showed significant baseline differences between groups in MOR BP (all p > 0.10), confirming that the observed binding changes were due largely to effects during treatment.
Long-Term Differential Changes in MOR BP Following Acupuncture and Sham Treatment
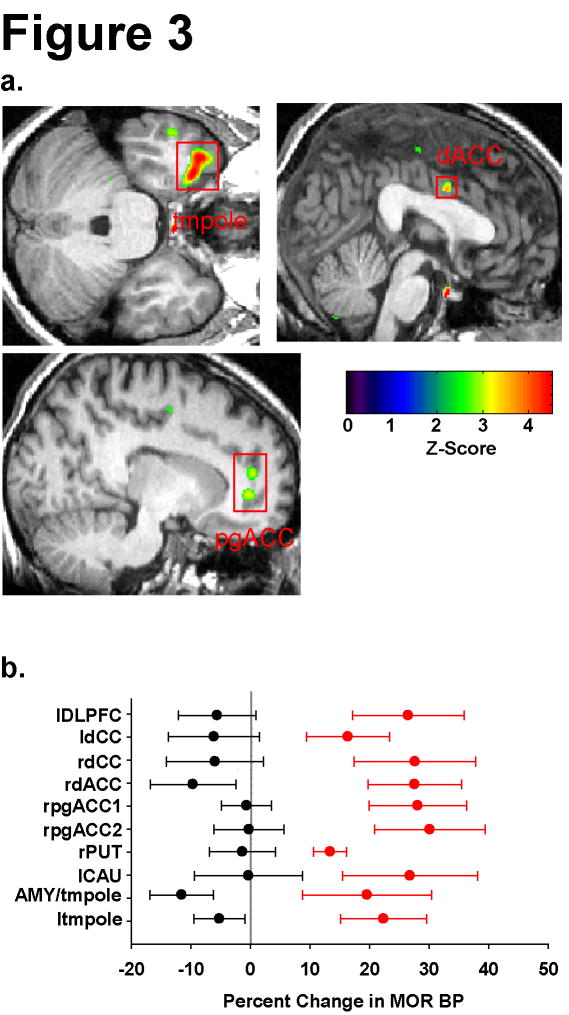
Long-term differences in MOR BP between TA and SA treatments were likewise examined with two separate comparisons: Comparison I = [TA(baseline2-baseline1) > SA(baseline2-baseline1)] and Comparison II = [SA(baseline2-baseline1) > TA(baseline2-baseline1)]. 10 regions were identified as having differences in MOR BP between groups with Comparison I (Table 2 and Figure 3). No regions were detected with Comparison II that met significance after correction for multiple comparisons (see Supplementary Figure 1B for glass brain results). Inspection of Table 2 and Figure 3 indicates that treatment differences were again largely attributable to increases in MOR BP following TA whereas SA evoked either small reductions in MOR BP or resulted in no change. Similar to the short-term effects, regions identified as showing increases in MOR BP included the amygdala, the cingulate cortex, the caudate, the putamen, and the temporal pole.
Table 2.
Regions Displaying Long-Term Increases in MOR Binding Following Acupuncture
| Region | MNI Coordinates | Voxels | Z | Acupuncture (TA) BP mean(s.e.m.) | Sham (SA) BP mean(s.e.m.) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Pre | Post | Pre | Post | ||||
| Glbl V | 0.62(0.03) | 0.65(0.03) | 0.58(0.03) | 0.59(0.04) | ||||||
| DLPFC | −30 | 29 | 26 | 351 | 3.3 | 0.63(0.04) | 0.80(0.09) | 0.79(0.11) | 0.74(0.10) | |
| ldCC | −3 | −16 | 57 | 225 | 3.3 | 0.90(0.06) | 1.02(0.05) | 0.92(0.11) | 0.82(0.07) | |
| rdCC | 10 | −8 | 54 | 192 | 3.1 | 0.76(0.07) | 0.94(0.08) | 0.71(0.05) | 0.67(0.06) | |
| dACC | 0 | 0 | 33 | 185 | 3.7 | 0.74(0.10) | 0.89(0.09) | 0.93(0.11) | 0.81(0.08) | |
| pgACC(1) | 13 | 42 | −1 | 628 | 3.3 | 0.92(0.08) | 1.16(0.10) | 0.87(0.06) | 0.87(0.08) | |
| pgACC(2) | 14 | 44 | 12 | 114 | 3.2 | 0.91(0.09) | 1.14(0.10) | 0.81(0.08) | 0.81(0.09) | |
| PUT | 20 | 12 | −12 | 158 | 3.2 | 1.47(0.07) | 1.66(0.07) | 1.48(0.08) | 1.45(0.08) | |
| CAU | −8 | 15 | 4 | 23 | 3.2 | 1.11(0.15) | 1.31(0.15) | 1.05(0.12) | 1.03(0.13) | |
| AMY/tmpole | −30 | 11 | −29 | 2417 | 4.8* | 1.08(0.08) | 1.24(0.09) | 1.27(0.12) | 1.09(0.07) | |
| tmpole | −39 | 15 | −28 | 1037 | 4.7* | 1.27(0.06) | 1.53(0.09) | 1.27(0.09) | 1.20(0.08) | |
p<0.05 corrected; all other regions p<0.001 uncorrected
Region Definitions: (Glbl V) global value; (DLPFC) dorsal lateral prefrontal cortex; (CC) cingulate cortex; (ACC) anterior cingulate cortex; (CAU) caudate; (PUT) putamen; (AMY) amygdala; (tmpole) temporal pole; (l: left; r: right; d: dorsal; pg: perigenual)
Figure 3.
Differential Long-Term Effects of Acupuncture and Sham Acupuncture on MOR Binding. A) Regions of interest showing increased MOR BP following acupuncture as compared to sham treatment. upper left: temporal pole (ltmpole), upper right: dorsal anterior cingulate cortex (dACC), lower left: two perigenual anterior cingulate regions (pgACC). B) Percent changes and S.E.M. in MOR BP (baseline2 –baseline1) for all regions identified. Red circles (TA) and black circles (SA) represent group mean values with standard error bars. Overall acupuncture resulted in an increase in MOR BP whereas sham treatment resulted in either no change or a decrease in binding ability.
To investigate whether the observed differences following TA and SA could be due to baseline differences, between treatment groups, we compared baseline MOR BP values for the above 10 regions. None of the ROIs showed significant baseline differences in MOR BP (all p > 0.15) again supporting the conclusion that they were largely due to effects following long-term treatment.
Changes in Clinical Pain
Clinical pain intensity was assessed prior to both PET imaging sessions. Significant reductions in pain were observed for the entire cohort for the total score of the Short Form of the McGill Pain Questionnaire (SF MPQ Total; mean diff(SD) treatment –baseline; −3.45(7.39), p<0.05) and trended towards significance for the sensory and pain affect subscales (Sensory Score:−2.65(5.98), p=0.06; Affective Score:−0.80(2.26), p=0.13). Both TA and SA resulted in clinically meaningful reductions in pain (SF MPQ Total Score mean diff(SD); TA:−4.00(6.72); SA:−2.90(8.33)), however there were no statistically significant differences in pain reduction between TA and SA (p>0.50).
Changes in MOR Binding are Associated with Changes in Clinical Pain
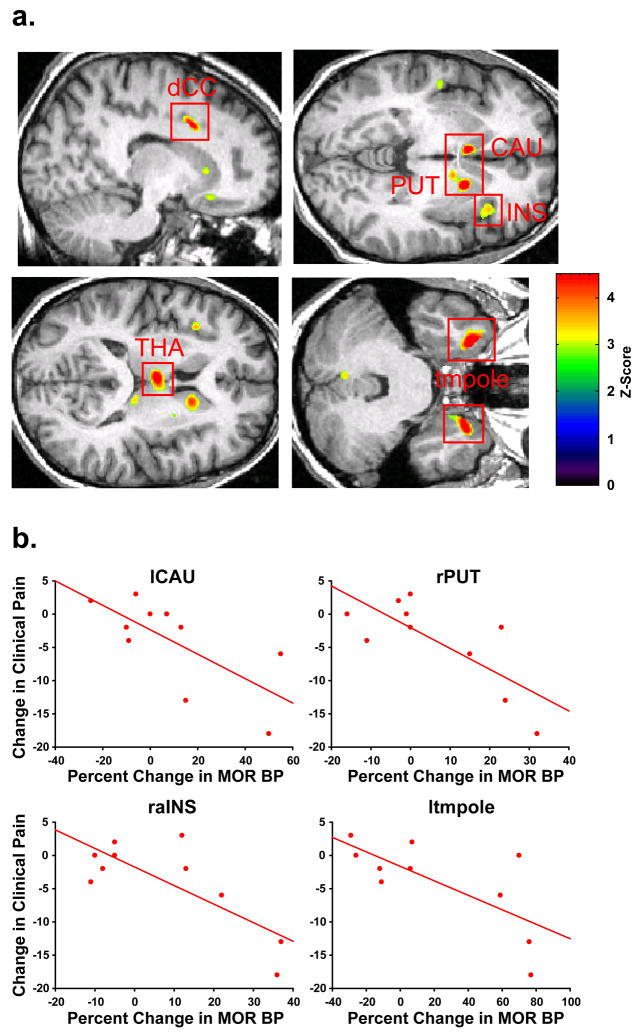
To investigate the analgesic relevance of the changes in MOR BP following TA, we correlated post – pre treatment changes in the SF MPQ Total score with the observed percent changes in MOR BP (i.e. baseline2 – baseline1) within participants that were treated with traditional acupuncture. Seven regions were identified as showing a negative correlation between changes in clinical pain and changes in MOR BP (Table 3 and Figure 4). Among these regions the thalamus, the cingulate, and the insula are known to play significant roles in processing and modulating pain sensations. Other regions included the caudate, the putamen and the temporal pole. These regions have been identified in other studies as showing differential response to acupuncture and sham treatment (Napadow et al., 2005; Hui et al., 2000). No regions were identified in the TA group as showing significant positive correlations between changes in MOR BP and changes in pain (see Supplementary Figure 1C for glass brain results). However the dorsolateral prefrontal cortex, which showed decreases in MOR BP in the SA group (see Figure 3) had a significant positive correlation with pain reduction following sham treatment (r=0.69; p=0.027). Individuals with greater reductions in MOR BP within this region, had greater reductions in clinical pain.
Table 3.
Regions Displaying Negative Correlation Between MOR Binding Changes and Changes in Clinical Pain Following Acupuncture
| Region | MNI Coordinates | Voxels | Z | r | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ldCC | −15 | 10 | 36 | 315 | 4.7 | −0.50 |
| raINS | 36 | 24 | −3 | 773 | 3.6 | −0.78 |
| lCAU | −7 | 10 | 2 | 686 | 6.8** | −0.70 |
| rPUT-rCAU | 19 | 7 | −4 | 2096 | 6.0** | −0.76 |
| lmTHA | −4 | −12 | 11 | 1008 | 5.4* | −0.65 |
| ltmpole | −29 | 15 | −34 | 1310 | 6.3** | −0.71 |
| rtmpole | 26 | 11 | −36 | 924 | 5.8** | −0.60 |
p<0.001 corrected;
p<0.005 corrected; all other regions p<0.05 corrected
Patients with greater increases in MOR BP displayed greater reductions in clinical pain
Figure 4.
Long-term Increases in MOR Binding following Acupuncture are Associated with Reductions in Clinical Pain. A) Regions of interest showing negative correlations between changes in MOR BP (baseline2 – baseline1) and changes in clinical pain (pain assessment2 – pain assessment1) following acupuncture. upper left: left dorsal cingulate cortex (ldCC), upper right: left caudate (lCAU), right putamen (rPUT), and right anterior insula (raIns), lower left: left medial thalamus (lmTHA), lower right: bilateral temporal pole (tmpole). B) Scatter plots of percent changes in MOR BP (post-pre) and changes in clinical pain (post-pre) for four regions depicted in A.
Assessment of Masking
To determine if the observed changes in MOR binding between groups could have resulted from participants knowing what treatment they received (i.e. unmasking of the trial), we asked all patients to guess what group they thought they were assigned to after the first PET imaging session. Participant guesses were consistent across the two groups. Four subjects in the TA group and three subjects in the SA group thought that they received traditional acupuncture. One participant in the SA group and two participants in the TA group thought that they received sham acupuncture, and four participants in the TA and six in the SA group did not know what treatment they received. These two distributions were not statistically different (Chi-Square value=0.88; p=0.65).
Discussion
We provide the first direct evidence of short- and long-term effects of acupuncture therapy on central MOR binding availability in chronic pain patients. Overall we find that traditional acupuncture therapy evokes an increase in MOR availability over both short and long periods. These changes were absent in sham treated patients where either no change was detected or decreases in MOR BP were observed. Reduction in central MOR BP during SA is consistent with increased endogenous opioid release during placebo administration (Zubieta et al., 2005; Scott et al., 2008). For both short- and long-term effects of TA, areas showing increases in BP included a number of brain regions classically implicated in the regulation of pain and stress in humans (Zubieta et al., 2001; Zubieta et al., 2003b), such as the amygdala, the dorsal and perigenual anterior cingulate, and the insular cortex. Other regions also shown to be involved in responses to pain and other salient stimuli and where TA induced significant effects on MOR BP included the nucleus accumbens, the caudate, and the putamen (Gear and Levine, 1995; Scott et al., 2006). The nucleus accumbens and the dorsal cingulate are both regions that we identified previously as showing reduced binding in FM patients as compared to controls (Harris et al., 2007). Finally, a region of the temporal pole showed increases in binding following TA for both short and long time periods, and displayed a significant negative correlation with changes in clinical pain. This temporal pole region has previously been identified as showing responsiveness to negative mood (Kennedy et al., 2006; Zubieta et al., 2003b) as well as acupuncture treatment (Napadow et al., 2005; Hui et al., 2000).
Our findings of widespread increases in regional MOR binding availability are consistent with a previous trial of acupuncture in rodents showing that acupuncture induces an increase in the number of central MOR binding sites following treatment (Gao et al., 1997). For changes that arise following long-term therapy, this could involve increased transcription and translation of MORs and their subsequent insertion into the plasma membrane. Indeed acupuncture treatment has been shown to modulate the levels of transcription factors within the central nervous system (Lao et al., 2004). However this explanation does not address the relatively rapid increases in MOR BP that we observe (i.e. within 45 minutes) following needle insertion. One possible explanation originates from animal and tissue preparations where increases in the plasma membrane expression of all three classes of opioid receptors have been shown occur in neurons following excitation. The sub-cellular localization of μ- (Browning et al., 2004), κ-(Shuster et al., 1999), and Δ- (Bao et al., 2003) opioid receptors all appear to be dynamically regulated by neural activity. Following neuronal excitation, all three classes of receptors have been shown to be trafficked to the plasma membrane within the time frame that we observe our short-term acupuncture effects (i.e. within 45 minutes). This type of regulation of glutamate receptors has been observed during long-term potentiation (LTP) and long-term depression (LTD) where neuronal activity modulates receptor expression at the plasma membrane (Malenka, 2003). Interestingly a recent study by Xing et al. suggests that acupuncture can also induce LTD in the spinal cord in a rat model of chronic pain and this depression is abolished by the opioid receptor antagonist naloxone (Xing et al., 2007). LTD-type modulation of MORs and subsequent changes in synaptic strength could function as a mechanism for acupuncture analgesia given the lasting effects of acupuncture observed here and in other clinical trials (Brinkhaus et al., 2006; Linde et al., 2005; Melchart et al., 2005; Witt et al., 2005).
Another intriguing result from the present study is that although MOR BP values were differentially altered by TA and SA, reduction in clinical pain was similar between groups. In a clinical trial, when an active treatment does not exhibit superior efficacy to a sham or placebo, the active treatment is assumed to be ineffective and only operating via a placebo effect. However this study suggests that this may be an erroneous conclusion. In this instance, our non-insertion sham procedure evoked a similar reduction in pain as our true acupuncture and we speculate that this occurred via a different mechanism. The analgesic effects of SA could have been due to regional reductions in MOR BP, consistent with activation of this class of receptors during placebo effects (Zubieta et al., 2005), whereas TA evoked an increase in receptor binding availability. This interpretation is entirely consistent with the observed positive correlation between decreases in MOR BP within the dorsolateral prefrontal cortex and decreased pain in the SA group. These reductions in MOR BP may also be operating in TA however these effects may be “masked” by the increases in receptor binding availability noted above.
Finally we explored the relationship between increases in MOR BP following acupuncture and subsequent changes in clinical pain. We found that many of the same regions showing increases in binding following acupuncture therapy were also associated with reductions in clinical pain. Since our previous study found reductions in MOR BP in FM patients (Harris et al., 2007), acupuncture may act to increase or “normalize” MOR binding ability in FM patients to levels that are more representative of pain free controls.
To determine if participants could tell the difference in treatments and unblind the trial, we asked our participants to guess which treatment they thought they received following the first PET imaging session. We found that both groups had similar guesses for their treatment assignments suggesting our results were not likely to be explained by participant knowledge of treatment assignment.
In this work some participants were taking medications, however we monitored closely their usage (see Supplementary Table 2). Patients remained on stable doses of existing medications for the entire duration of the study. Therefore, medications are unlikely to represent a confounding factor in the analyses presented. Any medication confound would be operative in both pre- and post-treatment scans as well as for TA and SA groups.
Our sham intervention was performed on non-acupuncture points and did not involve skin penetration. Therefore our differential effects of TA and SA may be due to point location and/or skin penetration. Future studies are needed to determine if the differential effects on MOR BP are due to either skin penetration or acupuncture point stimulation or a combination of both.
Overall our data strongly imply divergent opioid receptor mechanisms in acupuncture and sham acupuncture therapy. Although the fundamental mechanisms underlying these processes await further investigation, central opioid receptors appear to be involved in both treatments, albeit with differing effects within the same brain structures. Greater insight into these effects may be obtained in animal models of chronic pain disorders.
Supplementary Material
Acknowledgments
This work was funded by Department of Army grants DAMD (17/002-0018 to D.J.C. and W81XWH-07-2-0050 to R.E.H.) and National Institutes of Health (M01-RR000042 to R.E.H.; R01 AT 001415 to J.K.Z., K01 AT01111-01 to R.E.H., and K01-AT002166 and P01-AT002048 to V.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hokfelt T, Zhou Z, Zhang X. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M. The neurobiology of placebo analgesia: from endogenous opioids to cholecystokinin. Prog Neurobiol. 1997;52:109–125. doi: 10.1016/s0301-0082(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Biella G, Sotgiu ML, Pellegata G, Paulesu E, Castiglioni I, Fazio F. Acupuncture produces central activations in pain regions. Neuroimage. 2001;14:60–66. doi: 10.1006/nimg.2001.0798. [DOI] [PubMed] [Google Scholar]
- Brinkhaus B, Witt CM, Jena S, Linde K, Streng A, Wagenpfeil S, Irnich D, Walther HU, Melchart D, Willich SN. Acupuncture in patients with chronic low back pain: a randomized controlled trial. Arch Intern Med. 2006;166:450–457. doi: 10.1001/archinte.166.4.450. [DOI] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. μ-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:7344–7352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Geller EB, Adler MW. Electrical stimulation at traditional acupuncture sites in periphery produces brain opioid-receptor-mediated antinociception in rats. J Pharmacol Exp Ther. 1996;277:654–660. [PubMed] [Google Scholar]
- Dougherty DD, Kong J, Webb M, Bonab AA, Fischman AJ, Gollub RL. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav Brain Res. 2008;193:63–68. doi: 10.1016/j.bbr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995a;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995b;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gabilondo AM, Meana JJ, Garcia-Sevilla JA. Increased density of μ-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682:245–250. doi: 10.1016/0006-8993(95)00333-l. [DOI] [PubMed] [Google Scholar]
- Gao M, Wang M, Li K, He L. Changes of μ-opioid receptor binding sites in rat brain following electroacupuncture. Acupunct Electrother Res. 1997;22:161–166. doi: 10.3727/036012997816356662. [DOI] [PubMed] [Google Scholar]
- Gear RW, Levine JD. Antinociception produced by an ascending spino-supraspinal pathway. J Neurosci. 1995;15:3154–3161. doi: 10.1523/JNEUROSCI.15-04-03154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in μ-opioid receptor density in the brains of suicide victims. Brain Res. 1990;530:312–316. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central μ-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Tian X, Williams DA, Tian TX, Cupps TR, Petzke F, Groner KH, Biswas P, Gracely RH, Clauw DJ. Treatment of fibromyalgia with formula acupuncture: investigation of needle placement, needle stimulation, and treatment frequency. J Altern Complement Med. 2005;11:663–671. doi: 10.1089/acm.2005.11.663. [DOI] [PubMed] [Google Scholar]
- He LF, Lu RL, Zhuang SY, Zhang XG, Pan XP. Possible involvement of opioid peptides of caudate nucleus in acupuncture analgesia. Pain. 1985;23:83–93. doi: 10.1016/0304-3959(85)90233-7. [DOI] [PubMed] [Google Scholar]
- Ho WK, Wen HL. Opioid-like activity in the cerebrospinal fluid of pain patients treated by electroacupuncture. Neuropharmacology. 1989;28:961–966. doi: 10.1016/0028-3908(89)90196-2. [DOI] [PubMed] [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9:13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27:479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Jewett DM. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nucl Med Biol. 2001;28:733–734. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- Joshi A, Fessler JA, Koeppe RA. Improving PET receptor binding estimates from Logan plots using principal component analysis. J Cereb Blood Flow Metab. 2008;28:852–865. doi: 10.1038/sj.jcbfm.9600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang RX, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- Linde K, Streng A, Jurgens S, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes MG, Weidenhammer W, Willich SN, Melchart D. Acupuncture for patients with migraine: a randomized controlled trial. JAMA. 2005;293:2118–2125. doi: 10.1001/jama.293.17.2118. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Melchart D, Streng A, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes M, Hummelsberger J, Irnich D, Weidenhammer W, Willich SN, Linde K. Acupuncture in patients with tension-type headache: randomised controlled trial. BMJ. 2005;331:376–382. doi: 10.1136/bmj.38512.405440.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- Napadow V, Kettner N, Liu J, Li M, Kwong KK, Vangel M, Makris N, Audette J, Hui KK. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain. 2007;130(3):254–66. doi: 10.1016/j.pain.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24:193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert A, Dionne R, Ng L, Bragin E, Moody TW, Pert CB. Alterations in rat central nervous system endorphins following transauricular electroacupuncture. Brain Res. 1981;224:83–93. doi: 10.1016/0006-8993(81)91118-5. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endorphin implicated. Life Sci. 1976;19:1757–1762. doi: 10.1016/0024-3205(76)90084-9. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Sherman KJ, Hogeboom CJ, Cherkin DC, Deyo RA. Description and validation of a noninvasive placebo acupuncture procedure. J Altern Complement Med. 2002;8:11–19. doi: 10.1089/107555302753507140. [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. J Neurosci. 1999;19:2658–2664. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stux G, Hammerschlag R. Clinical Acupuncture: scientific basis. New York: Springer; 2001. [Google Scholar]
- Witt C, Brinkhaus B, Jena S, Linde K, Streng A, Wagenpfeil S, Hummelsberger J, Walther HU, Melchart D, Willich SN. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet. 2005;366:136–143. doi: 10.1016/S0140-6736(05)66871-7. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Xing GG, Liu FY, Qu XX, Han JS, Wan Y. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208:323–332. doi: 10.1016/j.expneurol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects μ-opioid neurotransmitter responses to a pain stressor. Science. 2003a;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic μ-opioid neurotransmission. Arch Gen Psychiatry. 2003b;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional μ-opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.