Abstract

The cyclization of γ,δ-unsaturated tertiary hydroperoxides in the presence of a palladium(II) catalyst afforded 1,2-dioxanes resembling biologically active natural products. A variety of substrates were screened, and synthetic manipulations were accomplished to construct compounds with structural similarity to antimalarial targets.
The discovery of peroxide-containing natural products as active agents against malaria1 and various cancers2 has led to an increased effort to synthesize these compounds and their derivatives.3 As a class, cyclic peroxides are the most common peroxide-containing motifs isolated.4 The structures in Figure 1 represent three important types of endoperoxides: 1,2-dioxolanes (as found in plakinic acid C);5 1,2,4-trioxanes (as represented by artemisinin);3a and 1,2-dioxanes (as exemplified by peroxyplakoric acid A1 methyl ester).6
Figure 1.
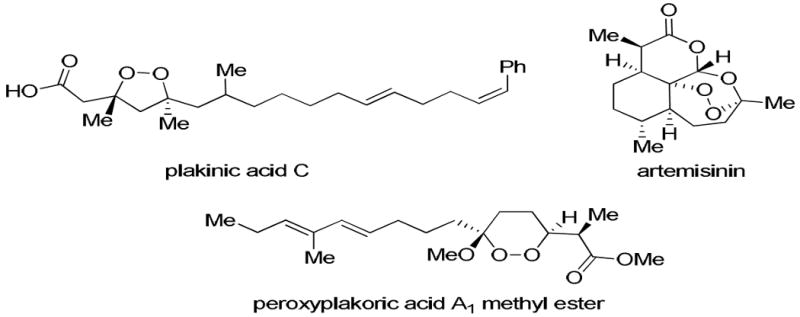
Cyclic Peroxide Natural Products
In light of the pharmaceutical potential of peroxides, the development of methods to prepare these compounds would aid in the discovery of new peroxide-containing drugs.1c The lability of the weak O–O bond makes installation and functionalization of peroxides particularly challenging, however.3d,7 The synthesis of 1,2-dioxanes has been accomplished in various ways. The intramolecular nucleophilic displacement of leaving groups, such as halides8 or mesylates9, has been employed. The use of peroxides as nucleophiles for the intramolecular attack on epoxides is another method for the synthesis of 1,2-dioxanes.10 While these methods are useful, they require preformation of the desired leaving group. A common strategy for the synthesis of endoperoxides is the cyclization of pendant hydroperoxides onto activated alkenes. For example, conjugate additions of peroxide nucleophiles onto electron-deficient alkenes have been used.11 These conditions also facilitate formation of epoxide side products, arising from Weitz-Scheffer oxidation,12 resulting in diminished yields of the desired endoperoxide. In addition, peroxyl radical cyclizations onto olefins are well established in the literature.13 Methods involving intramolecular attack of peroxide nucleophiles onto halonium14 and mercuronium ions,15 generated in situ from alkenes, have been used to yield 1,2-dioxanes. The potential drawback to these reactions is that residual iodine and mercury atoms may need to be removed by a subsequent transformation.15c
Given the large number of cyclic peroxides containing the 1,2-dioxane moiety, we sought to develop a complementary approach to synthesize this important structural motif. We reasoned that a late transition metal could combine alkene activation, intramolecular attack of the peroxide, and subsequent removal of the activating species in a single transformation. Related heteroatom nucleophiles, such as alcohols16 and amines,17,18 have been widely demonstrated to undergo cyclization onto olefins activated by electrophilic transition metal catalysts, including palladium(II) complexes. In contrast, only one example of a transition metal-catalyzed reaction resulting in peroxide-containing products has been reported.19 Nonetheless, the formation of isomeric products limits the reaction's utility. In this Letter, we report the palladium-catalyzed synthesis of cyclic peroxides that can be functionalized to give compounds structurally related to biologically active natural products.
Our first experiments were focused on the feasibility of catalyzing the intramolecular addition of hydroperoxides onto pendant olefins. The model substrate, unsaturated tertiary hydroperoxide 1a, was synthesized from the corrresponding alcohol.20 Next, hydroperoxide 1a was treated under Corey's conditions,19 but only decomposition products were observed. It was reasoned that a sacrificial oxidant could oxidize an intermediate palladium(0) species to prevent premature degradation of the free hydroperoxide. Addition of one equivalent of benzoquinone (BQ) gave a mixture of products including the desired 1,2-dioxane, as identified by 1H and 13C NMR spectroscopy. Alcohol 4a, which was likely formed by reduction of the peroxide, was observed, as was its cyclized product, furan 3a.16
Additional screening of reaction conditions with unsaturated hydroperoxide 1b provided a set of standard conditions for peroxycyclization. From these studies, it was clear that employing catalytic Pd(OAc)2 afforded higher conversions and yields than when using Pd(OCOCF3)2, [(NHC)Pd(allyl)Cl]2,21 Pd(PPh3)2Cl2, Pt(PPh3)2Cl2, or PdCl2. Exchanging NaH2PO4 with pyridine suppressed furan formation, simplifying isolation of the endoperoxide. In contrast to the success with benzoquinone, some oxidants (N-chlorosuccinimide, 2,3-dichloro-5,6-dicyanobenzoquinone, K2S2O8, O2, Re2O7, Ag2CO3/O2) gave mostly decomposition products, while others (HOAc/MnO2, cumene hydroperoxide, Ag2O) provided the desired 1,2-dioxane, albeit in lower yields. When used as an oxidant in 1,4-dioxane, the combination of catalytic benzoquinone and stoichiometric Ag2CO3 (or AgOAc)22 gave comparable yields to the reaction using stoichiometric benzoquinone (Scheme 2). Other viable solvents include toluene and 1,2-dichloroethane.23 As observed previously, reduction of peroxide 1b to alcohol 4b was a major side product of the reaction when 1,2-dichloroethane was used as the solvent. Using Ag2CO3 suppressed reduction, but promoted oxidation to other unidentified products.
Scheme 2.
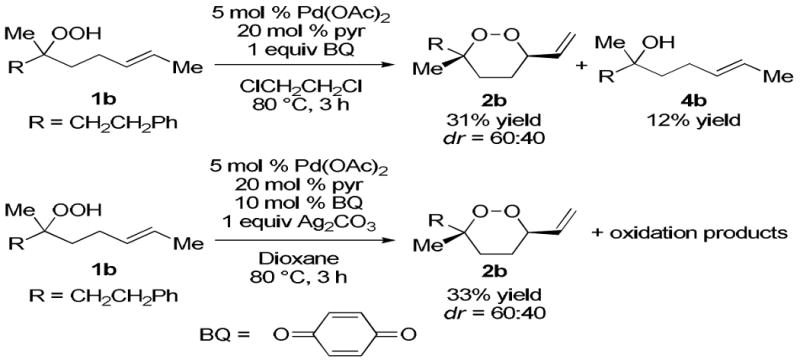
Optimized Reaction Conditions
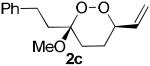
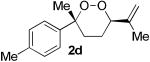
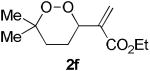
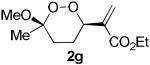
The application of this peroxypalladation to various unsaturated hydroperoxides is displayed in Table 1. Yields for these reaction are generally consistent among substrates. The alkyl tertiary hydroperoxides afforded modest diastereoselectivities, where the major diastereomer was assigned by comparing 13C NMR chemical shifts of the methyl group on the endoperoxide ring.24 Cyclization of mixed peroxyacetals (entries 2 and 6) gave higher diastereoselectivities, which could result from placing the methoxy group in the axial position due to the anomeric effect.25 Products that resemble known biologically active natural products, such as peroxyplakoric acid A1 methyl ester (Figure 1), were obtained from α,β-unsaturated esters (entries 5 and 6). The reaction appears to be specific for tertiary γ,δ-unsaturated hydroperoxides, because substrates with different substitution did not cyclize (entries 7 and 8).
Table 1.
Palladium-Catalyzed Cyclization of Unsaturated Peroxides
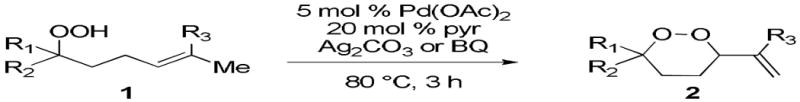 | |||
|---|---|---|---|
| entry | substrate | product | % yield (dr) |
| 1 |  |
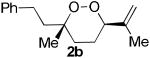 |
35 (75:25)a |
| 2 | 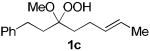 |
 |
34 (>97:3)b |
| 3 | 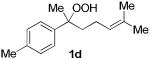 |
 |
30 (75:25)a |
| 4 | 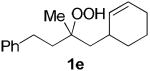 |
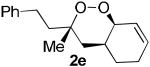 |
35 (48:30:16:6)a,c |
| 5 | 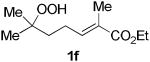 |
 |
31b |
| 6 | 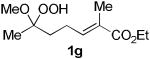 |
 |
30 (90:10)b |
| 7 | NRd | ||
| 8 | 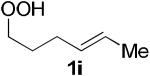 |
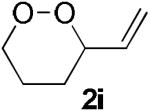 |
NRd |
Conditions A: 0.50 mmol substrate, 0.025 mmol Pd(OAc)2, 0.10 mmol pyridine, 0.050 mmol BQ, 1.0 mmol Ag2CO3.
Conditions B: 0.50 mmol substrate, 0.025 mmol Pd(OAc)2, 0.10 mmol pyridine, 0.50 mmol BQ.
dr of initial hydroperoxide = 1:1
NR = no product formed
A mechanism can be proposed by consideration of other reactions of peroxides with alkenes (Scheme 3).19,26,27 Ligand exchange of peroxide 1 for the acetate and coordination of the alkene would give peroxypalladium species 5.26 Syn-addition across the double bond and subsequent β-hydride elimination affords the 1,2-dioxane 2 and liberates a palladium(II) hydride, which reductively eliminates acetic acid.26,27 Reoxidation of the palladium(0) species with benzoquinone provides the requisite palladium(II) catalyst.28
Scheme 3.
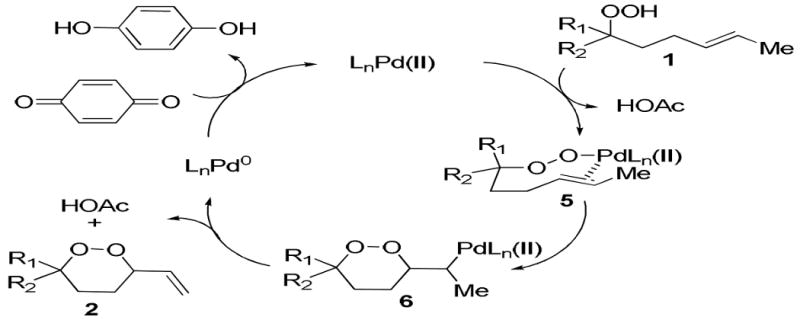
Proposed Mechanism for Peroxypalladation
The stability of cyclic peroxides allowed further manipulation of the products to provide structures that are analogous to those found in biologically active compounds (9). In particular, the 1,2-dioxane core with an acetic acid side chain is a common structural motif in naturally occuring peroxides, such as peroxyplakoric acid A1 methyl ester (Figure 1) and its derivatives. We were particularly interested in structures 2f and 2g because the ester functional group provided a convenient synthetic handle (Scheme 4). Given that many of the bioactive structures are saturated, hydrogenation of the C–C double bond is an essential transformation. Attempts to hydrogenate in the presence of Pd/C failed to produce the desired product, likely promoting cleavage of the O–O bond. A procedure using diimide, which was generated in situ, reduced the double bond smoothly to afford the saturated endoperoxide 7f in good yield.29,30 Hydrolysis to the carboxylic acid was achieved using 1 M LiOH in MeOH,31 and the chloroquine derived amide 8f was then formed using N-(3-dimethylaminopropyl)-N'-ethylcarbo-diimide hydrochloride (EDC). Endoperoxide 8f resembles the previously reported five-membered analog (9), which has demonstrated potent antimalarial activity against chloroquine-resistant strains of Plasmodium falciparum, the most virulent form of the malaria parasite.32,33
Scheme 4.
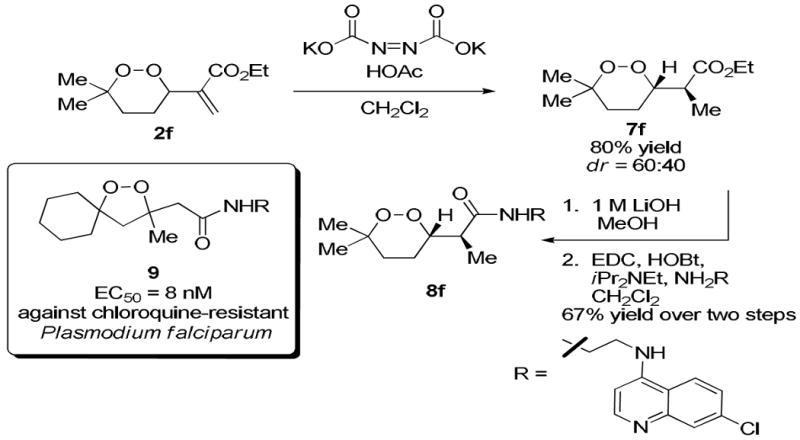
Synthetic Manipulation of Endoperoxide 2f
In conclusion, palladium-catalyzed cyclization of unsaturated tertiary hydroperoxides gives rise to 1,2-dioxane products. Following cyclization, further functionalization was achieved without degradation of the peroxide. This method is tolerant of functional groups that can be manipulated in subsequent transformations to afford biologically significant products.
Supplementary Material
Scheme 1.
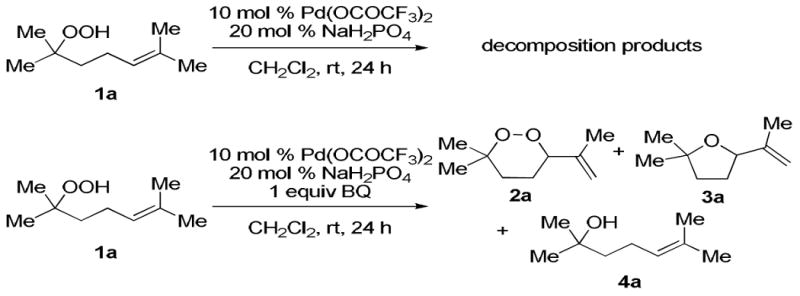
Initial Conditions for Peroxycyclization
Acknowledgments
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health (GM-61066), and the National Science Foundation (CHE-0315572). J.R.H. thanks Eli Lilly for a graduate fellowship. S.R.W. thanks the National Institute of General Medical Sciences for a postdoctoral fellowship (GM-085910). K.A.W. thanks Amgen and Eli Lilly for awards to support research. We would like to thank Dr. John Greaves and Ms. Shirin Sorooshian (UCI) for assistance with mass spectrometry and Dr. Phil Dennison (UCI) for help with NMR spectroscopy.
Footnotes
Supporting Information Available: Complete experimental procedures and product characterization. This material is available free of charge via the Internat at http://pubs.acs.org.
References
- 1.(a) Wu Y. Acc Chem Res. 2002;35:255–259. doi: 10.1021/ar000080b. [DOI] [PubMed] [Google Scholar]; (b) Jefford CW. Curr Med Chem. 2001;8:1803–1826. doi: 10.2174/0929867013371608. [DOI] [PubMed] [Google Scholar]; (c) Jefford CW. Curr Opin Investig Drugs. 2004;5:866–872. [PubMed] [Google Scholar]
- 2.(a) Woerdenbag HJ, Moskal TA, Pras N, Malingré MT, El-Feraly FS, Kampinga HH, Konings AWT. J Nat Prod. 1993;56:849–856. doi: 10.1021/np50096a007. [DOI] [PubMed] [Google Scholar]; (b) Efferth T. Drug Res Updates. 2005;8:85–97. doi: 10.1016/j.drup.2005.04.003. [DOI] [PubMed] [Google Scholar]; (c) Chen HH, Zhou HJ, Wang WQ, Wu GD. Cancer Chemother Pharmacol. 2004;53:423–432. doi: 10.1007/s00280-003-0751-4. [DOI] [PubMed] [Google Scholar]
- 3.(a) Klayman DL. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]; (b) Dembitsky VM, Gloriozova TA, Poroikov VV. Mini-Rev Med Chem. 2007;7:571–589. doi: 10.2174/138955707780859396. [DOI] [PubMed] [Google Scholar]; (c) Dembitsky V. Eur J Med Chem. 2008;43:223–251. doi: 10.1016/j.ejmech.2007.04.019. [DOI] [PubMed] [Google Scholar]; (d) McCullough KJ, Nojima M. Curr Org Chem. 2001;5:601–636. [Google Scholar]
- 4.(a) Casteel DA. Nat Prod Rep. 1999;16:55–73. [Google Scholar]; (b) Faulkner DJ. Nat Prod Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- 5.Horton PA, Longley RE, Kelley-Borges M, McConnell OJ, Ballas LM. J Nat Prod. 1994;57:1374–1381. doi: 10.1021/np50112a006. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Kondo K, Kitagawa I. Chem Pharm Bull. 1993;41:1324–1326. doi: 10.1248/cpb.41.1324. [DOI] [PubMed] [Google Scholar]
- 7.Dussault PH. Synlett. 1995:997–1003. [Google Scholar]
- 8.(a) Porter NA, Mitchell JC. Tetrahedron Lett. 1983;24:543–546. [Google Scholar]; (b) Porter NA, Gilmore DW. J Am Chem Soc. 1977;99:3503–3504. doi: 10.1021/ja00452a053. [DOI] [PubMed] [Google Scholar]; (c) Dussault PH, Zope UR. J Org Chem. 1995;60:8218–8222. [Google Scholar]
- 9.Ghorai P, Dussault PH, Hu C. Org Lett. 2008;10:2401–2404. doi: 10.1021/ol800657m. [DOI] [PubMed] [Google Scholar]
- 10.Xu XX, Dong HQ. J Org Chem. 1995;60:3039–3044. [Google Scholar]
- 11.(a) O'Neill PM, Searle NL, Raynes KJ, Maggs JL, Ward SA, Storr RC, Park BK, Posner GH. Tetrahedron Lett. 1998;39:6065–6068. [Google Scholar]; (b) Murakami N, Kawanishi M, Itagaki S, Horii T, Kobayashi M. Tetrahedron Lett. 2001;42:7281–7285. [Google Scholar]; (c) Murakami N, Kawanishi M, Itagaki S, Horii T, Kobayashi M. Bioorg Med Chem Lett. 2002;12:69–72. doi: 10.1016/s0960-894x(01)00673-4. [DOI] [PubMed] [Google Scholar]
- 12.Porter MJ, Skidmore J. Chem Commun. 2000:1215–1225. and references therein. [Google Scholar]
- 13.(a) Porter NA, Funk MO. J Org Chem. 1975;40:3614–3615. doi: 10.1021/jo00912a037. [DOI] [PubMed] [Google Scholar]; (b) Porter NA, Funk MO, Gilmore D, Isaac R, Nixon J. J Am Chem Soc. 1976;98:6000–6005. doi: 10.1021/ja00435a037. [DOI] [PubMed] [Google Scholar]
- 14.(a) Dussault PH, Davies DR. Tetrahedron Lett. 1996;37:463–466. [Google Scholar]; (b) Tokuyasu T, Masuyama A, Nojima M, McCullough KJ. J Org Chem. 2000;65:1069–1075. doi: 10.1021/jo991499m. [DOI] [PubMed] [Google Scholar]; (c) Kim HS, Begum K, Ogura N, Wataya Y, Tokuyasu T, Masuyama A, Nojima M, McCullough KJ. J Med Chem. 2002;45:4732–4736. doi: 10.1021/jm020208q. [DOI] [PubMed] [Google Scholar]
- 15.(a) Porter NA, Roe AN, McPhail AT. J Am Chem Soc. 1980;102:7574–7576. [Google Scholar]; (b) Porter NA, Zuraw PJ, Sullivan JA. Tetrahedron Lett. 1984;25:807–810. [Google Scholar]; (c) Porter NA, Zuraw PJ. J Org Chem. 1984;49:1345–1348. [Google Scholar]; (d) Bloodworth AJ, Curtis RJ, Mistry N. J Chem Soc, Chem Commun. 1989:954–955. [Google Scholar]
- 16.For examples of intramolecular hydroalkoxylation, see: Qian H, Han X, Widenhoefer RA. J Am Chem Soc. 2004;126:9536–9537. doi: 10.1021/ja0477773.Semmelhack MF, Bodurow C. J Am Chem Soc. 1984;106:1496–1498.Yang CG, Reich NW, Shi Z, He C. Org Lett. 2005;7:4553–4556. doi: 10.1021/ol051065f.Ohta T, Kataoka Y, Miyoshi A, Oe Y, Furukawa I, Ito Y. J Organomet Chem. 2007;692:671–677.Wolfe JP, Rossi MA. J Am Chem Soc. 2004;126:1620–1621. doi: 10.1021/ja0394838.Nakhla JS, Kampf JW, Wolfe JP. J Am Chem Soc. 2006;128:2893–2901. doi: 10.1021/ja057489m.
- 17.For examples of intramolecular hydroamination, see: Bender CF, Widenhoefer RA. J Am Chem Soc. 2005;127:1070–1071. doi: 10.1021/ja043278q.Fix SR, Brice JL, Stahl SS. Angew Chem Int Ed Engl. 2002;41:1641–1666. doi: 10.1002/1521-3773(20020104)41:1<164::aid-anie164>3.0.co;2-b.Michael FE, Cochran BM. J Am Chem Soc. 2006;128:4246–4267. doi: 10.1021/ja060126h.Liu Z, Hartwig JF. J Am Chem Soc. 2008;130:1570–1571. doi: 10.1021/ja710126x.Chianese AR, Lee SJ, Gagné MR. Angew Chem Int Ed Engl. 2007;46:4042–4059. doi: 10.1002/anie.200603954. and references therein.Ney JE, Wolfe JP. Angew Chem Int Ed Engl. 2004;43:3605–3608. doi: 10.1002/anie.200460060.Lira R, Wolfe JP. J Am Chem Soc. 2004;126:13906–13907. doi: 10.1021/ja0460920.
- 18.For cyclizations of hydroxylamines, see: Peng J, Lin W, Yuan S, Chen Y. J Org Chem. 2007;72:3145–3148. doi: 10.1021/jo0625958.
- 19.Yu JQ, Corey EJ. Org Lett. 2002;4:2727–2730. doi: 10.1021/ol0262340. [DOI] [PubMed] [Google Scholar]
- 20.Complete synthetic details are provided as Supporting Information. Peroxides can be explosive and appropriate safety measures should be taken (avoid light, heat and run on small scale).
- 21.NHC = N-heterocyclic carbene ligand (1,3-bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene).
- 22.(a) Giri R, Maugel N, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ. J Am Chem Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; (b) Li JJ, Mei TS, Yu JQ. Angew Chem Int Ed Engl. 2008;47:6452–6455. doi: 10.1002/anie.200802187. [DOI] [PubMed] [Google Scholar]
- 23.Calculation of the yield by NMR, based on an internal standard, affords a 41% yield of the observed 1,2-dioxane products. See Supporting Information for details. Resubjecting products to chromatographic conditions gave quantitative recovery of the product.
- 24.(a) Boukouvalas J, Pouliot R, Frechette Y. Tetrahedron Lett. 1995;36:4167–4170. [Google Scholar]; (b) He HY, Faulkner DJ, Lu HSM, Clardy J. J Org Chem. 1991;56:2112–2115. [Google Scholar]; (c) Capon RJ, Macleod JK, Willis AC. J Org Chem. 1987;52:339–342. [Google Scholar]
- 25.(a) Pierrot M, Idrissi ME, Santelli M. Tetrahedron Lett. 1989;30:461–462. [Google Scholar]; (b) Ichiba T, Scheuer PJ, Kelly-Borges M. Tetrahedron. 1995;45:12195–12202. [Google Scholar]
- 26.(a) Mimoun H, Charpentier R, Mitschler A, Fischer J, Weiss R. J Am Chem Soc. 1980;102:1047–1054. [Google Scholar]; (b) Roussel M, Mimoun H. J Org Chem. 1980;45:5387–5390. [Google Scholar]; (c) Mimoun H. Angew Chem Int Ed Engl. 1982;21:734–750. [Google Scholar]
- 27.(a) Nishimura T, Kakiuchi N, Onoue T, Ohe K, Uemura S. J Chem Soc, Perkin Trans I. 2000:1915–1918. [Google Scholar]; (b) Cornell CN, Sigman MS. J Am Chem Soc. 2005;127:2796–2697. doi: 10.1021/ja043203m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palladium(0)-catalyzed coupling of endoperoxides has been demonstrated: Xu C, Raible JM, Dussault PH. Org Lett. 2005;7:2509–2511. doi: 10.1021/ol050291m.
- 29.Adam W, Eggelte HJ. J Org Chem. 1977;42:3987–3988. doi: 10.1021/jo00444a055. [DOI] [PubMed] [Google Scholar]
- 30.The stereochemistry of the major diastereomer, as shown in Scheme 4, was assigned based on correlations of 1H and 13C chemical shifts to similar compounds reported in the literature: Ibrahim SRM, Ebel R, Wray V, Müller WEG, Edrada-Ebel R, Proksch P. J Nat Prod. 2008;71:1358–1364. doi: 10.1021/np800102u.
- 31.Herold P, Stutz S, Stojanovic A, Tschinke V, Marti C, Quirmbach M. 2005 PCT Int. Appl. #WO 2005070877. [Google Scholar]
- 32.Martyn DC, Ramirez AP, Beattie MJ, Cortese JF, Patel V, Rush MA, Woerpel KA, Clardy J. Bioorg Med Chem Lett. 2008;18:6521–6524. doi: 10.1016/j.bmcl.2008.10.083. [DOI] [PubMed] [Google Scholar]
- 33.The covalent attachment of the endoperoxide to a chloroquine moiety has been shown to enhance antimalarial activity as compared to either of these components individually: Walsh JJ, Coughlan D, Heneghan N, Gaynor C, Bell A. Bioorg Med Chem Lett. 2007;17:3599–3602. doi: 10.1016/j.bmcl.2007.04.054.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


