Figure 1.
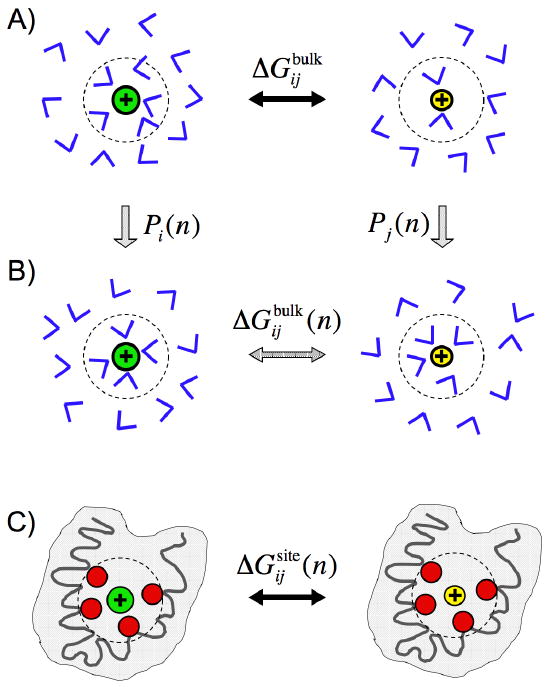
Schematic representation of the role of coordination states in bulk solvent in ion selectivity. In (A), the ions i and j are free in solution; the free energy difference is . In (B), the ions i and j are immersed in the bulk phase but constrained to be coordinated by n solvent molecules; the free energy difference is . The probabilities for ion i and j to spontaneously realize a n-coordinated state in solution are Pi(n) and Pj(n), respectively. In (C), the ions i and j are in the protein binding site surrounded by n ligands; the free energy difference is . Ion binding sites in proteins are typically embedded in a nonpolar low dielectric shell (colored grey) [45]. The notation is meant to emphasize that the binding site is effectively constrained to provide n coordinating ligands.
