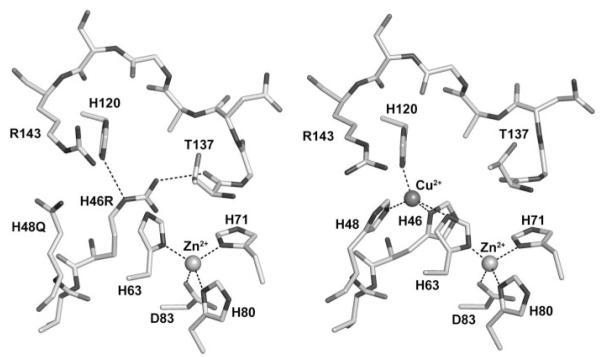
FIGURE 1. The metal-binding sites of the human FALS SOD1 double mutant H46R/H48Q (left) and the wild-type enzyme (right).
The structure surrounding the copper-binding site of one subunit of SOD1-H46R/H48Q is compared with SOD1-wt (right). All subunits in the crystal of SOD1-H46R/H48Q showed perturbed copper-binding sites. The structure shown is that of the crystal subunits that most closely resemble SOD1-wt. Arg-143 and residues corresponding to metal ions ligands in the wild-type enzyme (metal ligand 46, 48, 63, 71, 80, 83, and 120) are labeled. The metal ions are represented by spheres. Metal ligand and hydrogen bonds are shown as dotted lines. In the left image, the side chain of the Arg residue substituted at position 46 donates a hydrogen bond to the carbonyl oxygen of Thr-137 on the opposite side of the active site channel, preventing the binding of copper ion (see text).