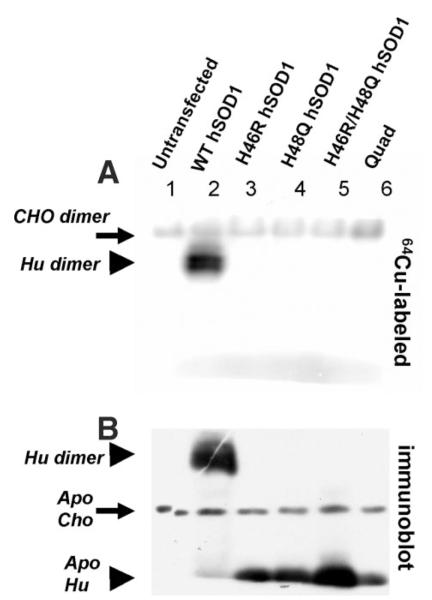
FIGURE 2. FALS mutations at copper-binding histidine residues of SOD1 dramatically reduce affinity for copper.
A, CHO cells were transfected to express human SOD variants before being metabolically labeled with 50 μCi/ml of 64Cu for 3 h. 100 μg of each cell lysate was separated on a non-reducing 10% polyacrylamide gel containing 0.1% SDS. The 64Cu autoradiogram shows Cu-labeled endogenous hamster SOD1 dimer (solid arrow) in all samples, and Cu-labeled human SOD1 dimer (solid arrowhead) only in the WT sample. B, a duplicate of the gel used for 64Cu autoradiogram was analyzed by SOD1 immunoblot, which reveals endogenous hamster SOD1 monomer and human SOD1 monomers that are not labeled by 64Cu. Note: apo refers to the absence or presence of copper.