FIGURE 3. FALS mutations at copper-binding histidine residues of SOD1 dramatically reduce the strength of normal dimer interactions.
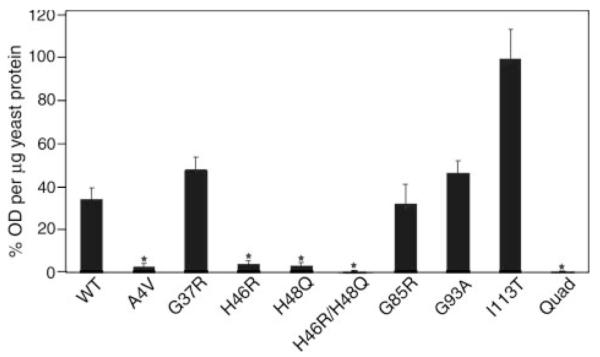
Interactions between the human WT SOD1 and mutant subunits were measured by a yeast two-hybrid assay (see “Experimental Procedures”). Yeasts expressing mutant-SOD1 bait-fusion proteins were mated with yeasts expressing wild-type SOD1 target-fusion proteins. The interactions between two subunits were recorded by induction of a β-galactosidase reporter gene. Enzyme activity was measured by optical density values per microgram of yeast protein extract and then normalized totheI113T,whichhadthehighestlevels ofβ-galactosidase production. The datarepresentmeans±S.E.Threeor fourindependentbaitfusion yeast colonies, all of which express sufficient proteins to pass a repression assay, were used for each SOD1 variant (total number of measurements for each variant, n = 9–12). Significance levels were determined by one-way analysis of variance with Bonferroni corrections; the asterisks mark interaction measurements that are significantly lower than that of the wild-type to wild-type interaction values and mutant to wild-type interaction values with p < 0.01.
