Abstract
Trillions of microbes inhabit the distal gut of adult humans. They have evolved to compete efficiently for nutrients, including a wide array of chemically diverse, complex glycans present in our diets, secreted by our intestinal mucosa, and displayed on the surfaces of other gut microbes. Here, we review how members of the Bacteroidetes, one of two dominant gut-associated bacterial phyla, process complex glycans using a series of similarly patterned, cell envelope-associated multiprotein systems. These systems provide insights into how gut, as well as terrestrial and aquatic, Bacteroidetes survive in highly competitive ecosystems.
Our distal gut is home to one of the most densely populated microbial ecosystems on Earth. Dominated by members of the domain Bacteria, the gut microbiota harbors a collection of beneficial symbionts (mutualists) that perform myriad functions, including the provision of metabolic attributes not encoded in the human genome. One such attribute is the ability to ferment otherwise indigestible complex glycans to products such as short-chain fatty acids that we can absorb (1). This microbial process can provide up to 10% of daily caloric intake depending upon the diet (2).
Viewed at the broadest taxonomic level, the distal gut microbiota of humans and other mammals is typically dominated by two of the ∼100 known bacterial phyla (divisions): Bacteroidetes and Firmicutes (3). During the past 2 years, several HMPs2 have been initiated throughout the world to better understand the assembly and composition of the microbiota in both healthy humans and those suffering from various pathophysiologic states such as obesity and inflammatory bowel diseases (4). These HMPs seek to determine the organismal diversity and gene content of the gut microbiota using culture-independent (metagenomic) approaches in conjunction with sequencing of several hundred cultured representatives of gut and non-gut communities. A central challenge to the sequencing efforts of HMPs is to go beyond descriptions of “who is there” or “what genes are present” in a community by continuing to probe the mechanisms by which microbes gain access to their habitats, operate as a community, and shape the biological properties of their hosts. In the distal gut, one important area is deciphering how community members have evolved to feed off the complex glycans that constantly inundate their habitat.
Individual representatives of several bacterial phyla, including the Bacteroidetes and Firmicutes, are capable of metabolizing a variety of complex carbohydrates (5). Early phenotypic surveys revealed that the Gram-negative Bacteroidetes typically harbor very broad saccharolytic potential, with some species able to target dozens of different complex glycans (6). Members of the genus Bacteroides are prominently represented in the intestine: some are notably aerotolerant and therefore readily cultured outside of their native habitat. Combined with the development of tools for their genetic manipulation (7), they have become favored models for characterizing mechanisms of glycan metabolism by gut bacteria.
A number of different plant-associated glycans are common components of our diets. These include plant cell storage glycans, such as starches and fructans, and plant cell wall glycans. Among cell wall glycans, cellulose is the most abundant, followed by two heterogeneous classes of polysaccharides: hemicelluloses and pectins. Hemicelluloses include xylan, galactoglucomannans, and xyloglucan (8). Pectin is composed of homogalacturonan and/or rhamnogalacturonan I backbones that can be decorated with additional side chains such as rhamnogalacturonan II, β-1,4- and β-1,3-galactans (each of which may contain α-arabinose branches), and α-arabinan (9). These dietary plant glycans, many of which cannot be digested in the proximal gut by the host, combine with mucin O-glycans, N-glycans, and glycosaminoglycans produced by the intestinal mucosa and the diverse repertoire of polysaccharide capsules and cell walls present on other gut microbes to form a biochemically rich nutrient foundation that sustains members of the distal gut microbiota.
Seminal work by members of the laboratory of Abigail Salyers provided a template for understanding how Bacteroides thetaiotaomicron, a prominent human gut Bacteroidete, is able to catabolize dietary glycans. Through their studies of starch degradation, they discovered a cell envelope-associated multiprotein system, which they named Sus (starch utilization system), that enables the bacterium to bind and degrade this carbohydrate. Subsequent microbial genome sequencing projects revealed that derivatives of this prototypic system (“Sus-like systems”) are highly represented in the genome of B. thetaiotaomicron and many other saccharolytic Bacteroidetes. A key feature of these Sus-like systems is the coordinated action of several gene products involved in substrate binding and degradation. Like other multicomponent strategies for glycan degradation (e.g. cellulosomes), this model highlights the fact that the concerted activities of multiple gene products can be more sophisticated and elaborate than their individual and isolated functions. Below, we review the current model of how the B. thetaiotaomicron Sus mediates starch utilization and then consider the breadth of different glycan substrates targeted by the many other Sus-like systems present in B. thetaiotaomicron and other related species from diverse habitats.
Prototypic Starch Utilization System (Sus)
Cellular Location of Sus Components
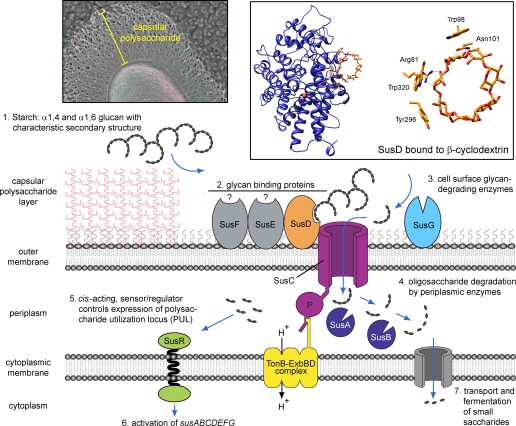
The sus locus consists of eight adjacent genes, susRABCDEFG, that encode proteins composing the cell envelope-associated apparatus illustrated in Fig. 1. SusCDEFG localize to the outer membrane (10). SusC is a member of the TonB-dependent receptor family, a group of outer membrane-spanning β-barrel proteins that transport solutes and macromolecules via energy derived from the proton-motive force and the TonB-ExbBD complex (11, 12). SusDEFG are predicted lipoproteins that contain a bacterial signal peptidase II recognition motif. Following cleavage of their signal peptides, an N-acyl-S-diacylglyceryl moiety is covalently linked to their N-terminal cysteines (13). Immunohistochemical studies of intact and disrupted B. thetaiotaomicron cells suggest that all four Sus lipoproteins are exposed to the external environment, consistent with the notion that they are trafficked to and remain at the outer leaflet of the plasma membrane (10). The three remaining Sus products (SusRAB) contain signal peptidase I recognition motifs. SusA and SusB remain in the periplasm (10), whereas SusR contains a single internal transmembrane region that allows it to span the cytoplasmic membrane and extend domains into both the periplasm and cytoplasm (see below).
FIGURE 1.
Functional model of glycan processing based on the eight-gene B. thetaiotaomicron starch utilization system (Sus). Individual starch processing steps are illustrated and numbered sequentially. Step 1, glycans transit through the surface capsular polysaccharide layer. The upper left inset shows a quick-freeze, deep-etch scanning electron micrograph of the capsule, highlighting its remarkably reticulated features (photograph courtesy of Robyn Roth and John Heuser). Step 2, glycans are bound by outer membrane-associated components such as SusD, which makes direct contacts with starch based on the three-dimensional structure of its helices. The upper right inset shows SusD binding to β-cyclodextrin (Protein Data Bank code 3CK8), a cyclic oligosaccharide that mimics the three-dimensional structure of starch. The arc of aromatic residues binding β-cyclodextrin is highlighted in yellow sticks, with the close-up view on the right displaying dashed lines for important hydrogen-bonding interactions. A single Ca2+ ion bound by SusD is shown as an orange sphere. Step 3, surface-bound glycans are degraded by outer membrane-associated glycoside hydrolases like SusG, generating smaller oligosaccharides that are transported across the outer membrane by SusC-like proteins. Step 4, oligosaccharides are degraded into their component mono- or disaccharides by periplasmic glycan-degrading enzymes such as SusA and SusB. Steps 5 and 6, liberated saccharides serve as signals for transcriptional regulators that activate PUL gene expression. Step 7, depolymerized sugars are imported across the cytoplasmic membrane.
Starch Catabolism by Sus
Initial genetic experiments suggested that Sus function was required only for catabolism of starch-derived glycans containing four or more glucose units and was dispensable during growth on glucose, maltose, and maltotriose (14). A requisite step in starch utilization is binding of the glycan to the cell surface (15). Before this can occur, extracellular starch must transit a polysaccharide capsule that may be several hundred nanometers thick (Fig. 1, upper left inset). Intriguingly, B. thetaiotaomicron coordinates expression of some of its eight capsular polysaccharide synthesis loci via transcriptional regulators that also mediate expression of a subset of its Sus-like systems (16). Thus, B. thetaiotaomicron may alter the chemical composition of its surface capsule to facilitate utilization of extracellular glycans.
Starch binding at the cell surface is accomplished through the concerted efforts of SusCDEF (10, 17). Recent biochemical and structural characterizations of SusD revealed that each SusD monomer has a single oligosaccharide-binding pocket that interacts with up to three individual glucose units in the target glycan (18). This pocket is composed of an arc of aromatic residues that conforms to the characteristic helical shape of amylose (Fig. 1, upper right inset) (19). Purified SusD binds to linear malto-oligosaccharides with relatively low affinity compared with cyclic oligosaccharides of the same length. For example, binding of linear maltoheptaose is almost 10-fold weaker than that of the cyclized form (18). In addition, the nature of the contacts between protein and ligand suggests that starch binding to SusD is driven by recognition of the backbone of the starch helices rather than by the stereochemistry of the individual glucose units. Thus, at least part of the substrate recognition mechanism of the Sus system depends on identifying the three-dimensional structure of the target substrate. This feature has also been observed for other carbohydrate-binding proteins that are not homologous to SusD, such as the carbohydrate-binding modules of numerous plant glycan-degrading glycoside hydrolases (19).
Genetic experiments revealed that SusD is required not only for utilization of larger starch-derived molecules with more than six glucose units but also for optimal growth on maltotetraose and maltopentaose, for which SusD has little to no detectable affinity (18). This suggests that SusD plays additional roles beyond polysaccharide binding, such as channeling malto-oligosaccharides to other Sus proteins or maintaining the structural integrity of a Sus protein complex.
Previous work in the Salyers laboratory indicated that both SusC and SusD are required for starch binding to the cell surface and interact with each other. Studies of isogenic mutants indicated that SusC and SusD alone contribute ∼60% of the starch binding affinity observed in wild-type B. thetaiotaomicron. Inclusion of SusE with SusC and SusD increases affinity for starch to >80% of the wild type. SusF contributes the remaining ∼20% (10). Recent experiments with purified SusE and SusF, which share ∼38% amino acid homology at their C termini, demonstrated that each interacts directly with starch and its oligosaccharides and that their affinity for starch-derived oligosaccharides is greater than that of SusD.3 Interestingly, experiments in which B. thetaiotaomicron was grown in vitro on highly purified and soluble forms of starch indicated that SusE and SusF are not essential for growth (20). These higher affinity starch-binding proteins may function to facilitate access to the more insoluble, granular forms of starch that reach the distal gut. Alternatively, they may sequester malto-oligosaccharides at the cell surface, rendering them less accessible to competing microbes.
Surface-bound starch is hydrolyzed through the action of an outer membrane α-amylase, SusG. Expression of SusG alone does not support growth on starch but is essential for starch utilization (17). Because all Sus functions are dispensable on substrates shorter than maltotetraose, it is likely that SusG, an endo-acting enzyme, generates internal cuts in a bound starch molecule, releasing oligosaccharides larger than maltotriose, which are then transported by SusC into the periplasmic compartment (Fig. 1).
In the periplasm, sequestered oligosaccharides are degraded into their component sugars prior to final transport to the cytosol. This is accomplished via two additional glycoside hydrolases, SusA and SusB, which possess neopullulanase and α-glucosidase activities, respectively (21, 22). Genetic disruption of either susA or susB alone does not eliminate growth on starch (21), suggesting that either enzyme is sufficient to depolymerize oligosaccharides, although it is possible that this function may be facilitated by other non-sus-associated enzymes (21). The atomic structure and enzymatic activity of SusB have been recently characterized (22). This enzyme prefers shorter substrates such as maltotriose, giving rise to the notion that SusA targets the larger oligosaccharides imported through SusC and that SusB works downstream from SusA (Fig. 1). Interestingly, SusB hydrolyzes substrates with different glucosidic linkages (α1,2, α1,3, α1,4, and α1,6). This “promiscuity” may increase the substrate spectrum for the Sus system, enabling use of substrates such as highly α1,6-branched pullulan.
Transcriptional activation of seven sus genes (susABCDEFG) is accomplished via a sensor/regulator, SusR, in the presence of starch. The N terminus of SusR is presumed to extend into the periplasmic space, whereas its C terminus, containing a predicted helix-turn-helix DNA-binding motif (23), remains in the cytoplasm (Fig. 1). The smallest fragment of starch that induces sus expression is the disaccharide maltose (23). Some portion of SusR must receive this signal, either directly or indirectly: this recognition is most likely mediated by its periplasmic domain. Maltose sensing would therefore occur prior to complete hydrolysis of malto-oligosaccharides to glucose and may provide additional sensory information to B. thetaiotaomicron, namely monosaccharide content and linkage. Such a strategy could help economize the cell's resources by allowing a more specific enzymatic response toward the sensed substrate.
Expansion of Sus-like Systems in Gut and Environmental Bacteroidetes
Sus-like PULs in B. thetaiotaomicron
The B. thetaiotaomicron genome contains 261 glycoside hydrolases and polysaccharide lyases currently annotated in the Carbohydrate-Active Enzymes (CAZy) Database (24). Remarkably, this organism's genome also contains 208 homologs of susC and susD, suggesting that the molecular strategy for starch utilization has been expanded to target other nutrients (25). There are 101 individual pairs of “susC-like” and “susD-like” genes, with the former always positioned immediately upstream of the latter (supplemental Fig. S1A). These susC/susD-like pairs are frequently components of larger gene clusters known as PULs that contain functions reminiscent of those in the prototypic sus, including glycan-degrading enzymes and regulators (26–28). Individual genes within sus-like PULs commonly share little or no homology with the prototypic sus locus beyond that of susC and susD. B. thetaiotaomicron contains 88 of these PULs, encompassing 866 genes and composing 18% of its genome (28). Although the minimum feature used to define each Sus-like PUL is a single pair of susC/susD homologs, 61% of these gene clusters in B. thetaiotaomicron resemble the prototypic sus locus in that they encode both glycan-degrading enzymes and a regulator.
Most sus-like PULs in B. thetaiotaomicron do not have regulators related to SusR but instead possess either hybrid two-component system phosphorelays or ECF-σ factor/anti-σ factor pairs. Like SusR, these regulators activate PUL transcription in response to glycans but do so through different mechanisms (supplemental Fig. S1, A and B) (28). A notable similarity that hybrid two-component system and ECF-σ/anti-σ regulators share with SusR is that each extends across the cytoplasmic membrane and equips the cell to sense both the sugar content and glycosidic linkages of glycan-derived saccharides before they are completely depolymerized (16, 29). This sensing may be through direct interactions with the saccharide or may be indirect and dependent on substrate recognition by other proteins. An example of regulation by indirect interaction of a transcription factor with saccharides is the activation of several dozen different B. thetaiotaomicron PULs by ECF-σ/anti-σ factor pairs through a mechanism termed trans-envelope signaling (16, 30). In this regulatory scheme, an inner membrane-spanning anti-σ factor interacts with a specialized SusC-like receptor in the outer membrane to relay the presence of extracellular glycans to a cytoplasmic ECF-σ transcription factor. The resulting protein bridge spans both cell membranes and likely facilitates a rapid transcriptional response when the appropriate glycan is present within the SusC-like receptor (see supplemental Fig. S1B and legend for further details) (16).
The physiological response to glycans sensed by PUL-associated regulators is contained in the adjacent PUL genes, which encode the enzymes required to degrade the sensed substrate. The predicted activities of the glycan-degrading enzymes contained in B. thetaiotaomicron vary widely among PULs, supporting the idea that different PULs have evolved to engage glycans besides starch. Indeed, whole-genome transcriptional profiles of B. thetaiotaomicron grown in vitro on purified glycans and in the distal gut of gnotobiotic mice consuming different diets (supplemental Fig. S1C) have revealed aspects of the “code” that links individual substrates to the activation of specific Sus-like PULs (27, 28, 31). For example, B. thetaiotaomicron deploys Sus-like PULs to target various plant-derived glycans, especially polygalacturonate, rhamnogalacturonan I, β-galactans, and α-arabinan contained in pectin.4 When dietary plant glycans are not available, host glycans (mucin O-glycans, N-glycans, and glycosaminoglycans) can serve as alternative nutrient sources (27, 28). In addition to harboring enzymes involved in breaking glycosidic linkages, some PULs also encode enzymes for removal of glycan modifications (e.g. the sulfatase and acetyl esterase depicted in supplemental Fig. S1A), which is likely a prerequisite step in degrading the underlying backbone.
Sus-like PULs in Other Bacteroidetes
The Bacteroidetes are a diverse and broadly distributed phylum and include members represented in both mammalian and insect (e.g. termite) gut, soil, and both fresh and salt water ecosystems (3, 32–34). They can be free-living or symbiotic and include endosymbionts (35, 36). Like their relatives in animal guts, a common feature associated with environmental Bacteroidetes is their ability to degrade complex glycans.
Several dozen complete or deep-draft genome sequences are now available for Bacteroidetes isolated from the animal gut or the environment. Most of these genomes encode sus-like PULs (28). The only exception noted to date is Candidatus Sulcia muelleri, an endosymbiont of hemipteran insects with a remarkably reduced genome size (∼250 kilobase pairs), reflecting its specialized intracellular habitat (35). The apparent diversity of Bacteroidetes Sus-like systems across this cosmopolitan phylum suggests that they have been adapted to degrade numerous substrates in diverse environments. Consistent with this idea, many Bacteroidetes species that harbor these systems metabolize additional substrates inaccessible to B. thetaiotaomicron: cellulose, hemicellulose, chitin, agarose, and alginate (6, 33, 34, 36).
Substrate diversity among Sus-like systems can be observed in both close and distant relatives of B. thetaiotaomicron. For example, unlike B. thetaiotaomicron, the human gut symbiont Bacteroides ovatus grows on all known plant hemicelluloses (6). This trait is likely due to the presence of additional PULs that target hemicelluloses: at least two of these target xylan and galactomannan (supplemental Fig. S1A) (37, 38). The SusD-like proteins encoded in these PULs, as well as lipoproteins of unknown function encoded by genes located downstream of these susD-like homologs (i.e.“susE-positioned” genes) (supplemental Fig. S1A), bind directly to their hemicellulose substrates.3 Interestingly, the lipoproteins encoded by these susE-positioned genes bear no homology to SusE and SusF yet appear to play a similar functional role in glycan binding. Proteins with similar signal peptidase II motifs are encoded in most PULs and are usually positioned downstream of the susD homologs (supplemental Fig. S1A), suggesting that they play important roles in the functions of Sus-like systems.
Additional evidence for expanded substrate diversity can also be observed outside of the Bacteroides genus. For example, the environmental Bacteroidete Flavobacterium johnsoniae contains multiple homologs of susC and susD, and these are also components of PULs. A notable feature of this organism is its ability to degrade the insoluble glycan chitin (39). Consistent with this phenotype, the F. johnsoniae genome harbors a PUL encoding three predicted chitinases (supplemental Fig. S1A). If this locus is shown to be responsible for chitin utilization, it would represent the first Sus-like system that targets a highly insoluble polysaccharide.
Prospectus
Each Bacteroidetes Sus-like system is a group of cell envelope-associated proteins that degrade a particular glycan. Like other microbial assemblies involved in nutrient degradation and uptake (e.g. cellulosomes), Sus-like systems highlight two key concepts: (i) multiple proteins work together during catalysis, and (ii) the genes encoding these concerted functions are often genomically linked into discrete clusters. The thousands of different sus-like PULs present in sequenced Bacteroidetes compose an amazingly diverse group of genes involved in regulating and directing glycan catabolism. Many of the genes in these clusters share little or no homology with genes contained in other PULs, and a substantial proportion (e.g. ∼39% of the 866 PUL genes in B. thetaiotaomicron) are not homologous to any genes of known function. Moreover, the breadth of substrates targeted by different PUL-containing Bacteroidetes suggests that the Sus-like paradigm has evolved to include a very broad suite of substrates, including those important to biofuel production, such as cellulose and hemicellulose. Pairing individual PULs with distinct glycan substrates will be a first step in defining the functions of these unknown genes and will likely lead to discovery of new proteins involved in glycan metabolism. Deciphering how these systems function promises to provide important insights into the dynamic operations of our gut microbiota and the foundations of our nutritional health. The lessons learned should also be applicable to other ecosystems that sustain our planet.
Acknowledgment
We thank Laura Kyro for help with graphics.
This work was supported, in whole or in part, by National Institutes of Health Grants DK30292, GM078800, HD07409, and AI073060. This work was also supported by the Missouri Life Sciences Trust Fund. This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
N. M. Koropatkin and T. J. Smith, unpublished data.
E. C. Martens, D. N. Bolam, and J. I. Gordon, unpublished data.
- HMP
- human microbiome project
- PUL
- polysaccharide utilization locus
- ECF-σ
- extracytoplasmic function-σ.
REFERENCES
- 1.Flint H. J., Bayer E. A., Rincon M. T., Lamed R., White B. A. (2008) Nat. Rev. Microbiol. 6, 121–131 [DOI] [PubMed] [Google Scholar]
- 2.McNeil N. I. (1984) Am. J. Clin. Nutr. 39, 338–342 [DOI] [PubMed] [Google Scholar]
- 3.Ley R. E., Hamady M., Lozupone C., Turnbaugh P. J., Ramey R. R., Bircher J. S., Schlegel M. L., Tucker T. A., Schrenzel M. D., Knight R., Gordon J. I. (2008) Science 320, 1647–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh P. J., Ley R. E., Hamady M., Fraser-Liggett C. M., Knight R., Gordon J. I. (2007) Nature 449, 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. (1977) Appl. Environ. Microbiol. 34, 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. (1977) Appl. Environ. Microbiol. 33, 319–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salyers A. A., Bonheyo G., Shoemaker N. B. (2000) Methods 20, 35–46 [DOI] [PubMed] [Google Scholar]
- 8.York W. S., O'Neill M. A. (2008) Curr. Opin. Plant Biol. 11, 258–265 [DOI] [PubMed] [Google Scholar]
- 9.Mohnen D. (2008) Curr. Opin. Plant Biol. 11, 266–277 [DOI] [PubMed] [Google Scholar]
- 10.Shipman J. A., Berleman J. E., Salyers A. A. (2000) J. Bacteriol. 182, 5365–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson A. D., Deisenhofer J. (2002) Biochim. Biophys. Acta 1565, 318–332 [DOI] [PubMed] [Google Scholar]
- 12.Schauer K., Rodionov D. A., de Reuse H. (2008) Trends Biochem. Sci. 33, 330–338 [DOI] [PubMed] [Google Scholar]
- 13.Bos M. P., Robert V., Tommassen J. (2007) Annu. Rev. Microbiol. 61, 191–214 [DOI] [PubMed] [Google Scholar]
- 14.Tancula E., Feldhaus M. J., Bedzyk L. A., Salyers A. A. (1992) J. Bacteriol. 174, 5609–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson K. L., Salyers A. A. (1989) J. Bacteriol. 171, 3192–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martens E. C., Roth R., Heuser J. E., Gordon J. I. (2009) J. Biol. Chem. 284, 18445–18457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves A. R., Wang G. R., Salyers A. A. (1997) J. Bacteriol. 179, 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koropatkin N. M., Martens E. C., Gordon J. I., Smith T. J. (2008) Structure 16, 1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boraston A. B., Bolam D. N., Gilbert H. J., Davies G. J. (2004) Biochem. J. 382, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho K. H., Salyers A. A. (2001) J. Bacteriol. 183, 7224–7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Elia J. N., Salyers A. A. (1996) J. Bacteriol. 178, 7173–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura M., Okuyama M., Tanzawa F., Mori H., Kitago Y., Watanabe N., Kimura A., Tanaka I., Yao M. (2008) J. Biol. Chem. 283, 36328–36337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Elia J. N., Salyers A. A. (1996) J. Bacteriol. 178, 7180–7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantarel B. L., Coutinho P. M., Rancurel C., Bernard T., Lombard V., Henrissat B. (2009) Nucleic Acids Res. 37, 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J., Mahowald M. A., Ley R. E., Lozupone C. A., Hamady M., Martens E. C., Henrissat B., Coutinho P. M., Minx P., Latreille P., Cordum H., Van Brunt A., Kim K., Fulton R. S., Fulton L. A., Clifton S. W., Wilson R. K., Knight R. D., Gordon J. I. (2007) PLoS Biol. 5, e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Bjursell M. K., Himrod J., Deng S., Carmichael L. K., Chiang H. C., Hooper L. V., Gordon J. I. (2003) Science 299, 2074–2076 [DOI] [PubMed] [Google Scholar]
- 27.Bjursell M. K., Martens E. C., Gordon J. I. (2006) J. Biol. Chem. 281, 36269–36279 [DOI] [PubMed] [Google Scholar]
- 28.Martens E. C., Chiang H. C., Gordon J. I. (2008) Cell Host & Microbe 4, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnenburg E. D., Sonnenburg J. L., Manchester J. K., Hansen E. E., Chiang H. C., Gordon J. I. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8834–8839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koebnik R. (2005) Trends Microbiol. 13, 343–347 [DOI] [PubMed] [Google Scholar]
- 31.Sonnenburg J. L., Xu J., Leip D. D., Chen C. H., Westover B. P., Weatherford J., Buhler J. D., Gordon J. I. (2005) Science 307, 1955–1959 [DOI] [PubMed] [Google Scholar]
- 32.Hongoh Y., Deevong P., Inoue T., Moriya S., Trakulnaleamsai S., Ohkuma M., Vongkaluang C., Noparatnaraporn N., Kudo T. (2005) Appl. Environ. Microbiol. 71, 6590–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie G., Bruce D. C., Challacombe J. F., Chertkov O., Detter J. C., Gilna P., Han C. S., Lucas S., Misra M., Myers G. L., Richardson P., Tapia R., Thayer N., Thompson L. S., Brettin T. S., Henrissat B., Wilson D. B., McBride M. J. (2007) Appl. Environ. Microbiol. 73, 3536–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer M., Kube M., Teeling H., Richter M., Lombardot T., Allers E., Würdemann C. A., Quast C., Kuhl H., Knaust F., Woebken D., Bischof K., Mussmann M., Choudhuri J. V., Meyer F., Reinhardt R., Amann R. I., Glöckner F. O. (2006) Environ. Microbiol. 8, 2201–2213 [DOI] [PubMed] [Google Scholar]
- 35.Moran N. A., Tran P., Gerardo N. M. (2005) Appl. Environ. Microbiol. 71, 8802–8810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allouch J., Helbert W., Henrissat B., Czjzek M. (2004) Structure 12, 623–632 [DOI] [PubMed] [Google Scholar]
- 37.Valentine P. J., Arnold P., Salyers A. A. (1992) Appl. Environ. Microbiol. 58, 1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead T. R. (1995) Biochim. Biophys. Acta 1244, 239–241 [DOI] [PubMed] [Google Scholar]
- 39.McBride M. J., Braun T. F., Brust J. L. (2003) J. Bacteriol. 185, 6648–6657 [DOI] [PMC free article] [PubMed] [Google Scholar]