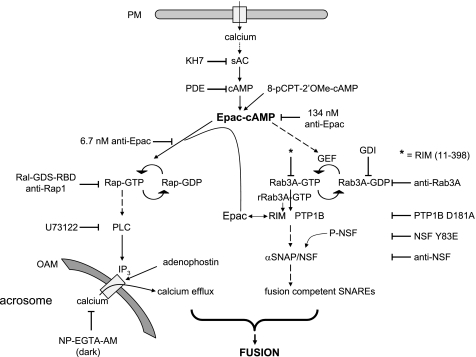
FIGURE 9.
Working model for the biochemical cascade leading to the AR. Calcium enters the cell from the extracellular milieu when channels open in the plasma membrane (PM) in response to progesterone or other physiological inducers, when a calcium ionophore transports it to the cytosol, or through the SLO-generated pores. Cyclic AMP synthesized by sAC (or exogenously added 8-pCPT-2′-O-Me-cAMP) activates Epac, which directly activates Rap1. In turn, active Rap1 stimulates the synthesis of IP3 through the stimulation of a PLC activity. This IP3 (or exogenously added adenophostin) elicits the efflux of calcium from an IP3-sensitive store (likely the acrosome). 2-APB blocks calcium efflux through IP3-sensitive channels (data not shown). Epac-cAMP also indirectly activates Rab3A, triggering the tethering of the acrosome to the plasma membrane through the assembly of large macromolecular complexes. A reaction taking place during or as a consequence of tethering initiates the activation and/or recruitment of PTP1B, which in turns activates NSF. Next, NSF/α-SNAP render SNARE proteins fusion-competent. Both a local increase in calcium coming from the acrosome through IP3-sensitive channels and SNAREs converge to accomplish the final steps of membrane fusion. Recombinant Rab3A (rRab3A-GTP) initiates the AR through an alternative pathway (gray) that presumably begins with the recruitment of RIM to achieve tethering. The large quantities of RIM recruited by recombinant Rab3A-GTP-γ-S bind Epac and the intracellular calcium pathway proceeds as described. OAM, outer acrosomal membrane. Solid arrows mean there is one step between the terms connected, and dashed arrows mean that the number of steps is either unknown or not depicted for simplicity.