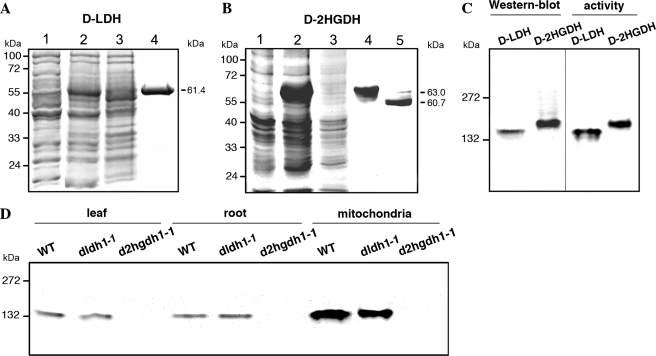
FIGURE 2.
A. thalianad-LDH and d-2HGDH analyzed by gel electrophoresis. Coomassie-stained SDS-PAGE of the progress of recombinant d-LDH (A) and d-2HGDH (B) purification. Lane 1, 20 μg of noninduced cell culture lysate. Lane 2, 20 μg of cell culture lysate after 2 h of induction with isopropyl β-d-thiogalactopyranoside. Lane 3, 20 μg of Ni2+-NTA column flow through. Lane 4, 5 μg of purified recombinant d-LDH or d-2HGDH. Lane 5, d-2HGDH after factor Xa digestion. The molecular masses of the purified proteins are indicated on the right. Molecular weight markers run in parallel are indicated on the left. C, native PAGE of recombinant d-LDH and d-2HGDH analyzed by Western blot using anti-His antibodies (on the left) and stained for activity using d-lactate and d-2HG as substrates in the case of d-LDH and d-2HGDH, respectively (on the right). The reaction was stopped after 30 min of incubation in the respective solutions. Molecular weight markers run in parallel are indicated on the left. D, native PAGE of leaf, root, and leaf mitochondria extracts from wild type (WT), dldh1-1, and d2hgdh1-1 analyzed for d-2HGDH activity using d-2HG as substrate. The reaction was stopped after 30 min of incubation. Molecular weight markers run in parallel are indicated on the left.