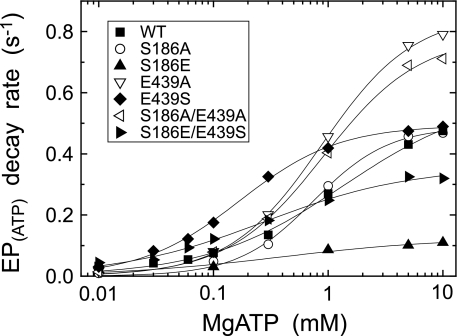
FIGURE 9.
MgATP dependence of the decay rate of E1PCa2 formed from ATP. E1PCa2 was first formed in 50 μl of a microsomes suspension in 10 μm [γ-32P]ATP, 10 μm Ca2+ (0.98 mm CaCl2 with 1 mm EGTA), and 0.1 m KCl, otherwise as in Fig. 4A. Phosphorylation was terminated by 100 μl of a buffer containing 8 mm EGTA, 1 μm A23187, 0.1 m KCl, 50 mm MOPS/Tris (pH 7.0), and various concentrations of ATP and MgCl2 (producing MgATP (more than 97% of the total ATP) with 6.2 mm free Mg2+). At different times after this MgATP addition, the decay reaction of E1PCa2 at 0 °C was quenched by acid. The rate of the single exponential decay of E1PCa2 obtained was plotted versus the MgATP concentration. Solid lines show the least squares fit to the Hill equation, and the parameters V0 (the rate at the lowest 10 μm MgATP), Vmax (the maximum rate), and K0.5 (MgATP concentration giving the half-maximal change) are given in Table 2.