Abstract
The sandcastle worm Phragmatopoma californica, a marine polychaete, constructs a tube-like shelter by cementing together sand grains using a glue secreted from the building organ in its thorax. The glue is a mixture of post-translationally modified proteins, notably the cement proteins Pc-1 and Pc-2 with the amino acid, 3,4-dihydroxyphenyl-L-alanine (DOPA). Significant amounts of a halogenated derivative of DOPA were isolated from the worm cement following partial acid hydrolysis and capture of catecholic amino acids by phenylboronate affinity chromatography. Analysis by tandem mass spectrometry and 1H NMR indicates the DOPA derivative to be 2-chloro-4, 5-dihydroxyphenyl-L-alanine. The potential roles of 2-chloro-DOPA in chemical defense and underwater adhesion are considered.
Keywords: 2-chloro-4,5-dihydroxyphenylalanine; Adhesive protein; Cement; Phragmatopoma californica; Sandcastle worm
Introduction
The marine sandcastle worm Phragmatopoma californica, like other sabellariids, gathers particulate debris including sand and shell fragments, and cements these together to build a tube-shaped dwelling (Fig. 1) [1,2]. The collective effort of many cohort worms leads to the construction of massive honeycombed mounds that approach reef-like dimensions in some coastal areas [3]. Even though Phragmatopoma is not a factor in the marine fouling of commercially important surfaces, the rapidity of tube-building and the versatility of its cement make the worm an ideal model system for studying both fundamental as well as practical aspects of marine adhesion [4–7].
Figure 1.
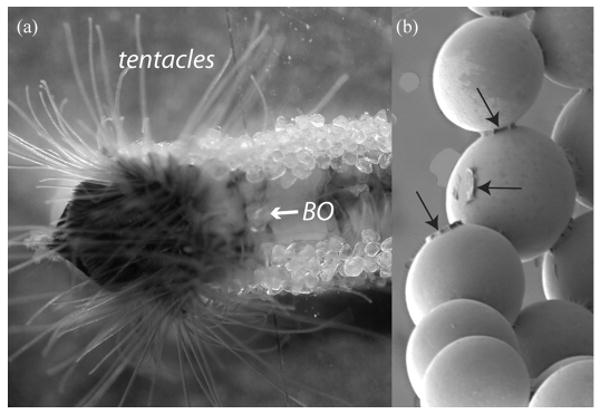
Tube production in the sandcastle worm Phragmatopoma californica supplied with clean sand and silica beads showing the anterior portion with extended sand collecting tentacles and building organ (BO). The zoom (right) highlights adhesive cement deposits (arrows) on silica beads (diameter 500 μm).
Marine adhesives have been classified into two basic types: temporary and permanent, each with several variations [8]. Phragmatopoma cement definitely qualifies as a “permanent” type of marine adhesive. Once it is put in place between two grains of sand, the cemented joint is expected to last. Previous studies of Phragmatopoma cement have characterized two groups of proteins—one that is strongly cationic and the other anionic at seawater pH [4–6]. The anionic protein contains a high mole% of phosphoserine (>40%), while the cationic protein is rich in lysine (20%). The positively charged proteins also contain nearly 10 mole% 3,4-dihydroxyphenyl-L-alanine (DOPA). Both DOPA and phosphoserine, which are post-translational modifications of tyrosine and serine, respectively, are considered to be crucial for the adhesive properties of the cement [5–7].
Two known activities of cement DOPA—cross-linking and adsorption—provide cohesiveness and stickiness, respectively. Adsorption, particularly chemisorption, of DOPA secures the adhesive proteins to surfaces [9]. On the other hand, cross-linking involves the formation of permanent covalent cross-links between protein chains and resembles “curing” in synthetic thermoset polymers. DOPA-dependent protein cross-linking is closely coupled to the redox potential of the DOPA-to-quinone half-reaction [10,11] since the quinone is the actual cross-linking species. DOPAquinone-derived cross-links in mussel adhesives include 5,5′-diDOPA and 5-S-cysteinyl-DOPA [12]. Cysteinyl-DOPA cross-links have been detected in tube-worm cement and are implicated in the cement curing process [6]. Histidine-DOPA cross-links can also occur according to a recent analysis of cephalopod beak [13], but have yet to be isolated from adhesive proteins. This study was undertaken to determine whether the o-diphenolic compounds detected in marine adhesives are limited to DOPA and the cross-links it forms. The discovery and characterization of halogenated DOPA in the cement of P. californica suggests otherwise.
Materials and Methods
P. Californica Tube Preparation
Colonies of P. californica were collected from the intertidal zone near Santa Barbara, CA, USA, and were maintained in the lab in circulating seawater tanks. Freshly made worm tubes were prepared and harvested as described before [5]. Worms were supplied with commercial sand (grain size diameters ranging between 400 and 600 μm from Sigma Aldrich, St. Louis, MO, USA). Newly built portions of the tubes were harvested every week without harming the worms. The collected tubes were washed extensively with deionized water followed by five washes of Milli-Q water. Cleaned tubes were briefly blotted with paper towels before being stored at−80°C.
Cl-DOPA Isolation
Lab-grown worm tubes (about 60 g) were washed, crushed, and dried prior to hydrolysis at 110°C in 6N HCl and 5% phenol in vacuo for 1 hr at which about 60–75% of the peptide bonds are cleaved. Longer hydrolysis times of tubes resulted in significantly reduced recovery of chloro-Dopa. The hydrolysate was flash evaporated and resuspended in 1 ml of 100 mM phosphate buffer (pH 7.5). The pH of resuspended sample was adjusted to 7.0 and centrifuged at maximum speed (15,000×g) on a benchtop centrifuge (MiniSpin, Eppendorf, Thermo Fisher Scientific, Pittsburgh, PA, USA) for 15 min. Because HCl is a potential cause for amino acid chlorination, comparison with an alternative nonchlorine-based hydrolysis method was also undertaken. A smaller amount of tubes was partially hydrolyzed in vacuo at 110°C in 4M methanesulfonic acid (MSA, Sigma-Aldrich Chemical, St. Louis, MO, USA) with 5% phenol for 1 hr in parallel with an HCl control. Since MSA cannot be eliminated by flash evaporation, the MSA hydrolysates were first neutralized with NaOH to pH 6.0 then further adjusted to 7.5 by adding 0.2 M Na2HPO4. The hydrolysate was then centrifuged at 15,000 rpm for 15 min in a bench-top centrifuge (MiniSpin, Eppendorf) to pellet insoluble fractions.
The neutralized hydrolysates were applied to a phenylboronate column (Affi-Gel 601 Boronate, Bio-Rad, Hercules, CA, USA) equilibrated with 100 mM phosphate at pH 7. To ensure efficient capture of DOPA and its o-diphenolic derivatives, the flow-through was reapplied to the column. Following binding, the column was washed sequentially with 10 column volumes (CVs) of 100 mM phosphate (pH 7.5), 10 CVs of 2.5 mM ammonium bicarbonate, and 10 CVs of Milli-Q water. The bound compounds, including a variety of derivatives related to DOPA, were eluted with 5% acetic acid: an aliquot was freeze-dried, resuspended in NaS buffer (Beckman Coulter, Fullerton, CA, USA), and subjected to amino acid analysis. The remainder of the eluant was further purified by reverse phase HPLC (Varian ProStar, Walnut Creek, CA, USA) system using a C-18 column (OD-300, Aquapore octadecyl, Perkin-Elmer, Shelton, CT, USA). HPLC elution buffers were H2O/0.1% TFA (trifluoroacetic acid) and acetonitrile/0.1% TFA with a gradient of 0% acetonitrile (ACN) from 0–5 min, 0% ACN from 5–50 min, 15% ACN from 50–55 min, 40% ACN from 55–70 min, and 100% ACN from 70–100 min. Fractions absorbing at both 220 and 280 nm were further analyzed by amino acid analysis and tandem mass spectrometry.
Amino Acid Analysis
Aliquots of key fractions eluting from phenylboronate and reverse phase HPLC, as well as a small amount of standard 3-chloro, 4,5-dihydroxyphenylalanine (3-Cl-DOPA) sample donated by the National Institutes of Mental Health Chemical Synthesis and Drug Supply Program, were freeze-dried and resuspended in NaS buffer (Beckman Coulter). The samples were analyzed on a Beckman 6300 Autoanalyzer (Beckman Coulter) using an elution program designed for separating post-translationally modified amino acids.
Tandem Mass Spectrometry
Another set of aliquots from phenylboronate, and reverse phase HPLC, was freeze-dried and resuspended in 50% ACN with 0.2% formic acid in water. These and the standard 3-Cl-DOPA samples were analyzed on a Waters Micromass QTOF2 tandem mass spectrometer (Waters, Milford, MA, USA) with electrospray ionization source and a Masslynx data system. Samples were infused into the source via a Harvard Apparatus model 22 syringe pump (Holliston, MA, USA) set at a flow rate of 5 μL/min. Capillary voltage was set at 3.5 kV for the positive ion mode and cone voltage was set at 45 V. MS spectra were collected using the TOF mass analyzer with a 1 s scan time. The TOF mass analyzer was tuned to a resolution of 10,000 (m/dm). Tandem MS spectra were collected following collision-induced decomposition using Ar as collision gas at a collision voltage set between 10–30 V during the data acquisition process.
1H NMR Analysis
Between 150–200 g (blotted wet weight) of new tubes were required to purify enough of the cement-derived Cl-DOPA using the methods described above for proton NMR. The cement-derived Cl-DOPA (100 μg) and standard 3-Cl-DOPA (200 μg) from NIMH were dissolved in 600 μL of 5% CD3COOD in D2O and run on a Avance DMX500 MHz SB NMR spectrometer (Bruker, Fremont, CA, USA). Data acquisition time was dependent on sample concentration. Trimethylsilane was used as an external standard.
Results
Based on a full HCl hydrolysis of protein in newly constructed tubes of the sandcastle worm, the cement represents less than 0.002% of the dry weight of the tubes. DOPA constitutes about 2 mole% of the cement [6]. Phenylboronate affinity chromatography has proven ideal for the selective capture of DOPA and its derivatives from complex mixtures of cement-derived amino acids [6,13]. Affinity chromatography combined with reversed phase HPLC is sufficient to purify some unusual derivatives from hydrolyzed sandcastle worm cement (Fig. 2 with inset). When HPLC fractions corresponding to the peak and shoulder at #28–29 are subjected to electrospray ionization mass spectrometry (Fig. 3), two features in the mass spectrum immediately stand out as atypical of biological molecules: a pair of doublets separated by 2 Da at m/z 291/289 and 234/232. These are strongly suggestive of organochlorine chemistry since Cl consists of two isotopes (mass 35.45 and 37.45) with a natural abundance ratio of 3:1. Fragmentation of the 289 peak by tandem MS produces primarily the imino a1 ion (m/z 186) of chloro-DOPA-Gly (Fig. 4a). The peak at m/z 150 is likely to be the carbocation derived from 186 ion after loss of HCl. Tandem MS of the 232 peak also produces the 186 ion (Fig. 4b) and is consistent with monochloro DOPA, although, without NMR, assignment of the chloro position is not possible with confidence. Indeed, MS/MS of authentic 3-chloro-4,5-dihydroxyphenylalanine (3-Cl-DOPA) produced a fragmentation (not shown) that was virtually identical with purified cement-derived chloro-DOPA, although the two compounds exhibit significantly different elution times by amino acid analysis (Fig. 5).
Figure 2.
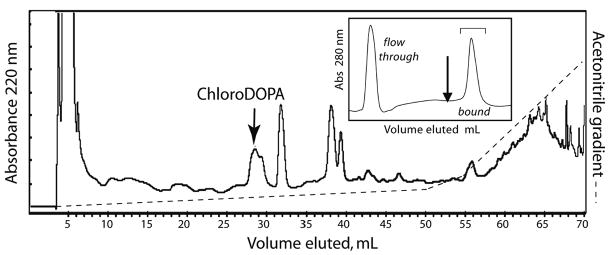
Purification of Cl-DOPA from acid-hydrolyzed sandcastle worm cement. Hydrolysates of cement are either flash-evaporated and redissolved in phosphate buffer (HCl hydrolysis) or neutralized with NaOH (MSA hydrolysis) and applied to phenylboronate (inset). As shown, there is an unbound component that flows through as well as bound material that can be eluted by 5% (v/v) acetic acid (arrow, inset). The bound fractions are pooled and applied to an HPLC column of C-18, from which Cl-DOPA elutes at about 28 min (arrow, flow 1mL/min). Gradient conditions are specified in Methods. Only the elution profile for 220 nm is shown.
Figure 3.
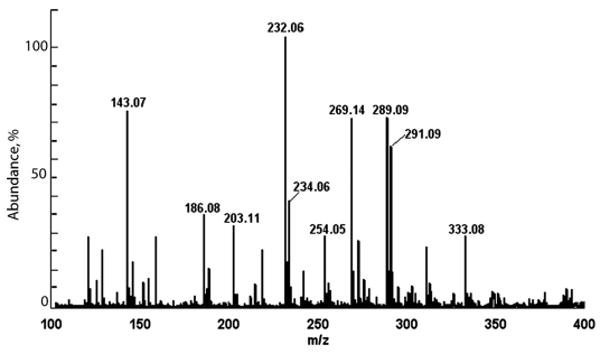
Electrospray ionization mass spectrum of phenylboronate affinity-captured and C-18 HPLC purified Cl-DOPA. In fractions #28–29, mass variants suggestive of the natural occurrence of Cl isotopes occur at m/z 232 and 289.
Figure 4.
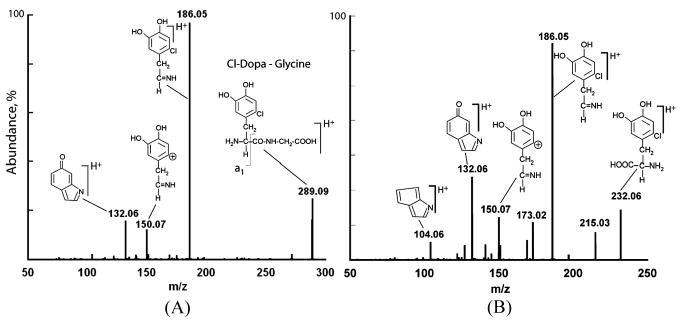
(A), Fragmentation of m/z 289. (B), Fragmentation of the m/z 232 peak.
Figure 5.
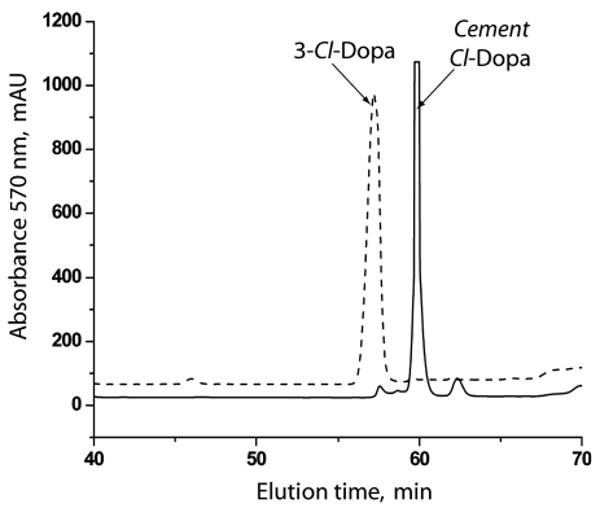
An amino acid analysis profile of a 3-Cl-DOPA standard versus the HPLC purified cement-derived chloroDOPA.
Ring assignment of the chloro group was deduced from coupling constants of the aromatic protons in cement-derived Cl-DOPA subjected to 1H NMR. There are three possible isomers of monochloro-DOPA: 2-,3-, or 6-Cl-DOPA. Each has only two ring protons that are para, meta, or ortho coupled, respectively. The coupling constants (JAB) between HA and HB (AB-type arrangement) were compared for both cement-derived Cl-DOPA and authentic 3-Cl-DOPA. The aromatic protons in the 1H NMR spectrum of the 3-Cl-DOPA standard exhibit two resonances that are further split into doublets. The coupling constant (JAB =JBA = 1,5 Hz) of these doublets corresponds to that of a meta-coupled system (Fig. 6). The coupling constant for ortho coupled protons in 6-Cl-DOPA would be considerably larger than 5 Hz [14]. In contrast, the cement-derived Cl-DOPA showed no peak splitting at chemical shifts δ = 6.9706 and 6.823 (Fig. 6) suggesting that Ha and Hb are located para to each other. This would place the chloro group at the 2-position, i.e., 2-chloro-4,5-dihydroxyphenylalanine (2-Cl-DOPA).
Figure 6.
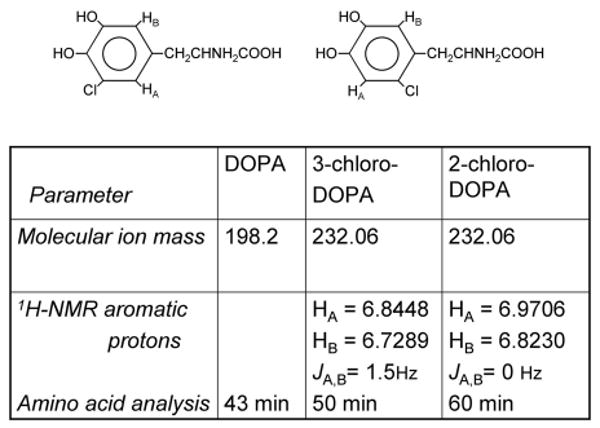
Comparison of NMR chemical shifts and coupling constants for (left) the aromatic protons of 3-Cl-DOPA and (right) cement-derived Cl-DOPA, identified as 2-Cl-DOPA.
Detection of significant 2-Cl-DOPA in Phragmatopoma cement raised concerns about whether it is present in the native material or is produced during cement hydrolysis in HCl. The latter possibility was eliminated by hydrolyzing cement in methanesulfonic acid (MSA). Equivalent amounts of Cl-DOPA were recovered from MSA hydrolysates. However, a 1 h-hydrolysis in MSA produced 10-fold better yields of DOPA than HCl (Fig. 7). This is a noteworthy point of concern for future determinations of DOPA content in crude materials. The relative molar proportion of DOPA:Cl-DOPA in the cement was 10:1 after MSA hydrolysis and 1:1 after HCl hydrolysis (N = 3 each treatment; SD ± 20% of the mean). Possibly DOPA is more readily released from the cement by MSA. Given that mature cement contains about 2 mole % DOPA, the DOPA: Cl-DOPA ratios tentatively suggest that 2-Cl-DOPA may be present at between 0.2 to 2 mole%. No other isomers of monoCl-DOPA were detected after either type of hydrolysis.
Figure 7.
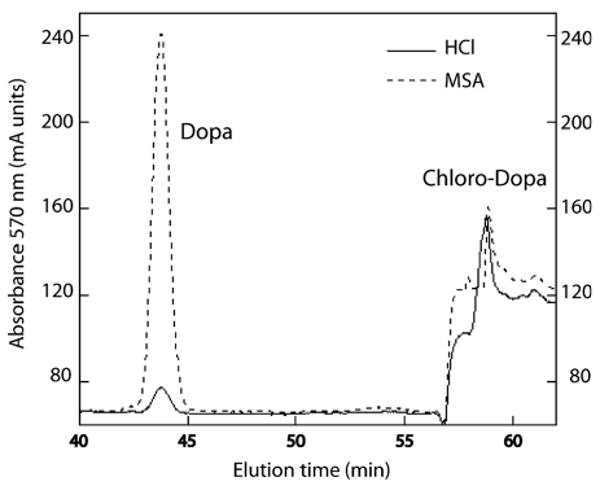
Comparison of MSA and HCl hydrolysis on the yields of DOPA and 2-Cl-DOPA recovered from sandcastle worm cement. The two methods showed no difference in Cl-DOPA yields, but DOPA yields appeared to be dramatically different.
Discussion
Protein-bound DOPA is crucial to marine adhesion for both (A) cohesive and (B) adsorptive properties [15] (Fig. 8). A fuller mechanistic understanding of its roles is frustrated by the complexity of DOPA chemistry). Phenylboronate affinity capture of DOPA and DOPA derivatives released from Phragmatopoma cement by mild hydrolysis opens the way to unraveling some of this complexity. In previous work, the cross-link 5-S-cysteinylDOPA was captured by phenylboronate for characterization from hydrolyzed Phragmatopoma cement and proposed to arise from a nucleophilic attack on DOPAquinone by the thiolate group of cysteine [12]. In the present study, 2-Cl-DOPA and the related peptide, 2-Cl-DOPA-glycine, have been identified as a major capture target by phenylboronate. The Cl-DOPA is not an artifact of hydrolysis and its detection in the dipeptide Y-G is consistent with the frequency of the Y-G sequence, which occurs at least 33 times in the cement precursor protein Pc-1, e.g., repeat consensus V-G-G-Y-G-Y-G-G-K-K and another 15 times in Pc-2 [6].
Figure 8.
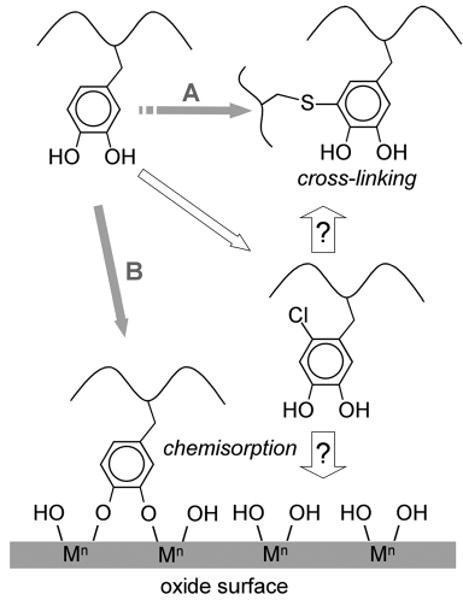
Pathways of DOPA reactivity in the cement proteins of P. californica. A. DOPA residues in Pc-1 and Pc-2 are oxidized to DOPAquinones, whereupon they react with cysteinyl residues in the same or other proteins to form cysteinyl-DOPA cross-links. B. The catecholate ion of DOPA chemisorbs to a metal (M) oxide shown in a bidentate binuclear complex. The 2-halogenation of DOPA residues in the proteins is a new pathway that could amplify either A or B, but B seems more likely.
There are over 4,000 known natural organohalogens and most of them occur in marine organisms [16]. We know of no previous report of a naturally occurring Cl-DOPA or, for that matter, Br- or I-DOPA. The closest known chemistry involves the halogenated tyrosines and dityrosines of various scleroprotein structures including ragworm jaws, insect cuticle, gastropod operculum, and horseshoe crab carapace [17–20]. A systematic mechanical analysis of ragworm jaws was unable to find any correlation between protein halogenation and mechanical properties [17]; therefore the function of this modification of tyrosine is still under active investigation.
What advantage could the presence of 2-Cl-DOPA confer on cement? Before speculating, it is worth stressing that sandcastle worm cement is not homogeneous, but consists of three very distinct functional domains—an outer skin, a porous interior, and an adhesive interface [5,21]. We still don't know how the highly variable precursor cement proteins Pc-1, Pc-2, and Pc-3 are distributed within these domains. A halogenated DOPA-containing protein in the outer skin might make it less prone to microbial fouling and degradation, as some of the best naturally occurring antifoulants and feeding deterrents are halogenated [16,22]. Chlorocatechols have acute toxic effects on microbes with a mode of action that involves narcosis and uncoupling of oxidative phosphorylation [23]. As the toxicity of low molecular weight chlorocatechols is tied to their ability to diffuse across biological membranes, the potential toxicity of chlorinated catechols tethered to a polymer backbone, e.g., protein, must await further analysis.
For DOPA-containing proteins at the interface between the cement and a mineral particle, for example, DOPA halogenation may offer another advantage. On metal oxide surfaces, DOPA provides an interaction between itself and the metal that exceeds all noncovalent interactions in water. The interaction energy between tethered DOPA and a TiO2 surface, for example, approximates −130 kJ/mole— roughly half the energy of a covalent bond [9]. DOPA, however, is prone to oxidize to DOPAquinone at the pH of seawater (pH 8.2) and also at acidic pH, when redox active metals such as FeIII are present [24]. The quinone can coordinate metal ions, but it is far inferior to DOPA as a ligand for most metal ions. Due to electron withdrawing effects, chlorination of catechols lowers the pKas of the phenolic OH groups and also lowers their redox potential, thus, making oxidation to quinone more difficult [25]. Chlorination of the 2 position of DOPA, for example, reduces the first phenolic pKa by ∼0.5 below that of DOPA (Table 1). Why cement prefers 2-Cl-DOPA over the 3- and 6-Cl-DOPA isomers remains unclear.
Table 1.
Effect of Chlorination on the pKa of the o-Dihydroxyl Groups in DOPA (pKa was Calculated using www.chemaxon.com/demosite/marvin/index.html)
| pK1 | pK2 | |
|---|---|---|
| DOPA | 12.50 (4-OH) | 8.74 (3-OH) |
| 6-Cl-4,5-DOPA | 12.05 (4-OH) | 7.82 (3-OH) |
| 3-Cl-4,5-DOPA | 7.85 (4-OH) | 12.01 (5-OH) |
| 2-Cl-4,5-DOPA | 8.32 (4-OH) | 12.15 (5-OH) |
4-Chlorocatechol is a close analogue of 2-Cl-DOPA and its adsorption on titanium oxide surfaces has been investigated by ATR FTIR spectroscopy and thermodynamic mass balance. Adsorption was highest between pH 7 to 8, and the structure of the adsorbed species was deduced to be a bidentate binuclear complex (for example, Fig. 8B) between the catechol and TiIV and possessing a 60% ionic and 40% covalent bonding character [26]. Thus, it seems reasonable to conclude that 2-Cl-DOPA would be as good as DOPA for strong surface interactions (Fig. 8). Indeed, Avdeef et al. [27] have proposed that more acidic catechols are useful auxiliary ligands for metals in that, given their lower pKas, they can stabilize metals by coordination at lower pHs. This stabilization could serve to protect the less acidic catechols like DOPA, whose interaction with metal ions is weaker at lower pH, from oxidation by metals. Given the acid-base properties of many hydrated metal oxide surfaces with acidic isoelectric points [28], similar arguments would pertain to adsorption.
Acknowledgments
This work was supported and funded by a grant from NIH (R01DE018468). The authors would also like to thank J. Pavlovich from the UCSB Chemistry Department and J. Hu from the Materials Research Lab for their help with mass spectrometry and NMR analysis, respectively.
Footnotes
One of a Collection of papers honoring J. Herbert Waite, the recipient in February 2009 of The Adhesion Society Award for Excellence in Adhesion Science, Sponsored by 3M.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Jensen RA, Morse DE. J Exp Mar Biol Ecol. 1984;83:107–126. [Google Scholar]
- 2.Vovelle J. Arch Zool Exp Gen. 1965;106:1–187. [Google Scholar]
- 3.Simmons SA, Zimmer RK, Zimmer CA. J Marine Res. 2005;63:623–643. [Google Scholar]
- 4.Waite JH, Jensen RA, Morse DE. Biochem. 1992;31:5733–5738. doi: 10.1021/bi00140a007. [DOI] [PubMed] [Google Scholar]
- 5.Stewart RJ, Weaver JC, Morse DE, Waite JH. J Exp Biol. 2004;207:4727–4734. doi: 10.1242/jeb.01330. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Sun CJ, Stewart RJ, Waite JH. J Biol Chem. 2005;280:42938–42944. doi: 10.1074/jbc.M508457200. [DOI] [PubMed] [Google Scholar]
- 7.Sun CJ, Fantner GE, Adams J, Hansma PK, Waite JH. J Exp Biol. 2007;210:1481–1488. doi: 10.1242/jeb.02759. [DOI] [PubMed] [Google Scholar]
- 8.Flammang P, Santos R, Haesaerts D. In: Progress in Molecular and Subcellular Biology – Subseries Marine Molecular Biotechnology. Matranga V, editor. Springer-Verlag; Berlin Heidelberg: 2005. pp. 201–220. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Scherer NF, Messersmith PB. Proc Natl Acad Sci. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haemers S, Koper GJM, Frens G. Biomacromol. 2003;4:632–640. doi: 10.1021/bm025707n. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Zhang Z, Cheng G, Dong S. Electroanal. 2002;15:103–107. [Google Scholar]
- 12.Sagert J, Sun CJ, Waite JH. In: Biological Adhesives. Smith AM, Callow JA, editors. Ch 7. Springer-Verlag; Berlin, Heidelberg: 2004. pp. 125–143. [Google Scholar]
- 13.Miserez A, Schneberk T, Sun CJ, Zok FW, Waite JH. Science. 2008;319:1816–1819. doi: 10.1126/science.1154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waite JH, Andersen SO. Biol Bull. 1980;158:164–173. [Google Scholar]
- 15.Waite JH, Holten-Andersen N, Jewhurst S, Sun CJ. J Adhesion. 2005;81:297–317. [Google Scholar]
- 16.Gribble GW. Prog Chem Org Nat Prod. 1996;68:1–423. doi: 10.1007/978-3-031-26629-4_1. [DOI] [PubMed] [Google Scholar]
- 17.Birkedal H, Khan RK, Slack N, Broomell C, Lichtenegger HC, Zok FW, Stucky GD, Waite JH. Chem Bio Chem. 2006;7:1392–1399. doi: 10.1002/cbic.200600160. [DOI] [PubMed] [Google Scholar]
- 18.Andersen SO. Acta Chem Scand. 1972;26:3097–3100. doi: 10.3891/acta.chem.scand.26-3097. [DOI] [PubMed] [Google Scholar]
- 19.Hunt S, Breuer SW. Biochim Biophys Acta. 1971;252:401–405. doi: 10.1016/0304-4165(71)90021-3. [DOI] [PubMed] [Google Scholar]
- 20.Welinder BS, Roepstorff P, Andersen SO. Comp Biochem Physiol. 1976;53B:529–533. doi: 10.1016/0305-0491(76)90212-1. [DOI] [PubMed] [Google Scholar]
- 21.Stevens MJ, Steren RE, Hlady V, Stewart RJ. Langmuir. 2007;23:5045–5049. doi: 10.1021/la063765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg PD, de Nys R, Kjelleberg S. Biofouling. 1998;12:227–244. [Google Scholar]
- 23.Schweigert N, Hunziker RW, Escher BI, Eggen RIL. Environ Toxicol Chem. 2001;20:239–247. [PubMed] [Google Scholar]
- 24.Mentasti E, Pelizzeti E, Saini G. J Chem Soc Dalton Trans. 1973;23:2690–2614. [Google Scholar]
- 25.Proudfoot GM, Ritchie IM. Aust J Chem. 1983;36:885–894. [Google Scholar]
- 26.Martin ST, Kesselman JM, Park DS, Lewis NS, Hoffmann MR. Environ Sci Technol. 1996;30:2535–2542. [Google Scholar]
- 27.Avdeef A, Sofen SR, Bregante TL, Raymond KN. JACS. 1978;100:5362–5370. [Google Scholar]
- 28.Bolger JC. In: Adhesion Aspects of Polymeric Coatings. Mittal KL, editor. Plenum Publishing; New York: 1983. pp. 3–18. [Google Scholar]


