Figure 2.
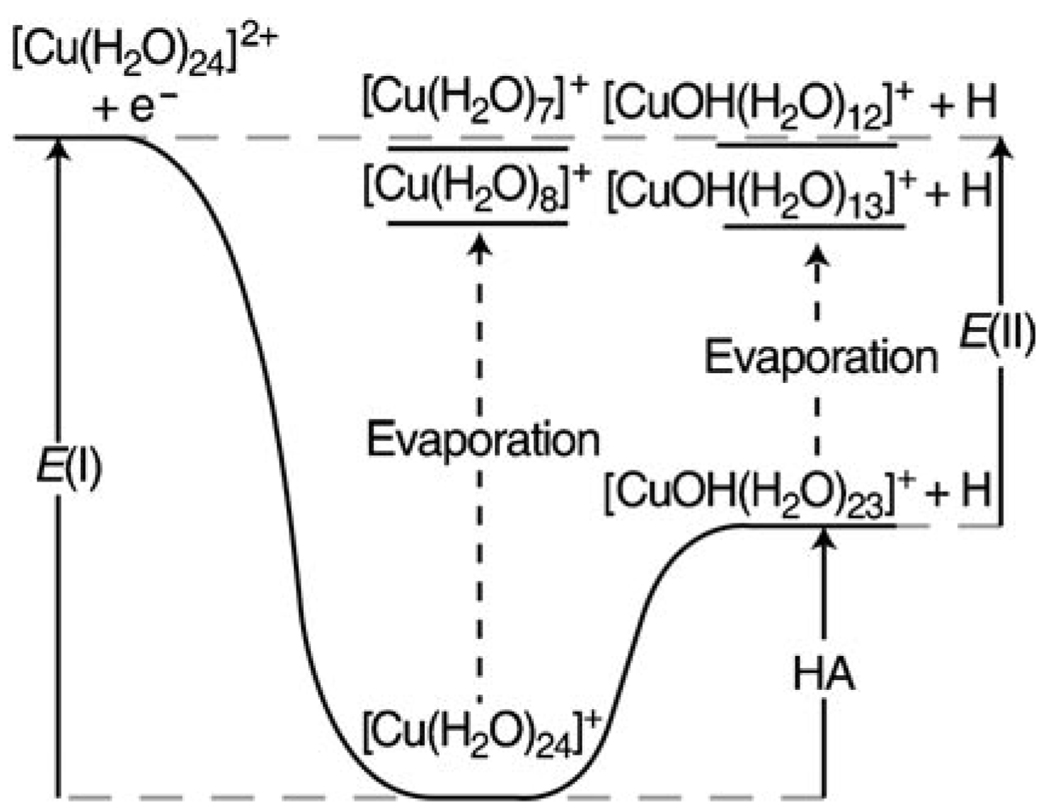
The recombination energy, E(I), and the energy corresponding to the formation of [CuOH(H2O)23]+ +H, E(II), is obtained from the average number of water molecules that evaporate for each pathway. The hydrogen atom affinity, HA, of the [CuOH(H2O)23]+ cluster is the difference between E(I) and E(II). The top grey dashed line indicates the energy of the products and reactants; the bottom grey dashed line indicates the energy of the reduced precursor at the same temperature of the precursor. The black dashed lines indicate the evaporation of water molecules from the reduced clusters.