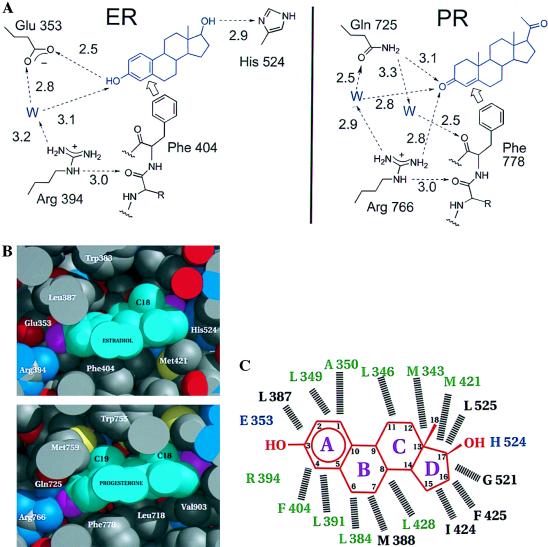
Figure 3.
Specificity determinants of the hormone-binding site specifies 3-hydroxy vs. 3-keto steroids. (A) The hydrogen-bonding network as seen in the hERαLBD and hPRLBD (18). The discriminating relationship of Glu/Gln to the 3-hydroxy/keto of the steroid is supported by a network of water-mediated hydrogen bonds involving the side chain of a conserved Arg and backbone carbonyl of a conserved Phe that, in turn, are fixed by hydrophobic contacts with the steroid ring. Note that the PR has no obvious hydrogen bonding discrimination at the 20-keto position of progesterone comparable to the hydrogen-bonding interaction seen between the 17-hydroxyl of estradiol and His-524. (B) Space-filling representation of estradiol in the ligand-binding pocket of hERαLBD and progesterone in the ligand-binding pocket of hPRLBD (18). For the proteins, carbon atoms are gray, oxygen atoms red, sulfur atoms yellow and nitrogen atoms blue. For the hormones, carbon atoms are cyan, oxygen atoms magenta. Drawn with midas (36). (C) Schematic of estradiol in the hERαLBD ligand-binding pocket in the structure shown here; hormone (red) rings are lettered, and carbon atoms are numbered. Residues hydrogen bonded directly to the hormone are blue. Dashed lines indicate hydrophobic van der Waals contacts with the hormone. Residues conserved among the steroid receptors are green, and variable residues are black. Residues contributed by helix 12 (1) are not shown.