Abstract
Klotho is an anti-aging protein with different functions of the full-length membrane protein and the secreted hormone-like form. Using overexpression and knock-down approaches as well as embryonic fibroblasts of knock-out mice we present evidence that Klotho is shedded by the α-secretases ADAM10 and 17 as well as by the β-secretase BACE1. The remaining membrane-bound fragment is a substrate for regulated intramembrane proteolysis by γ-secretase. Our data suggest that therapeutic approaches targeting these proteases should be carefully analyzed for potential side effects on Klotho-mediated physiological processes.
Keywords: Klotho, gamma-secretase, ADAM protease, BACE
1. Introduction
Klotho (Kl) is a protein directly linked to aging. Transgenic mice with an insertion in the Kl locus were found to develop drastic symptoms of premature aging starting after 3-4 weeks [1]. Symptoms included shortened life span, arteriosclerosis, osteoporosis, skin atrophy and emphysema and resemble human aging. On the contrary, transgenic mice overexpressing Kl have an extended life span, suggesting that it has anti-aging properties [2]. Kl is a 120-135 kDa type-I transmembrane protein [for review see 3]. Interestingly, Kl is shedded and the extracellular part is released into CSF and serum [4]. Kl is predominantly expressed in the kidney in distal convoluted tubules and in brain in the choroid plexus, with weaker expression in pituitary, hippocampus, parathyroid and others [1].
Kl seems to be involved in Vitamin D dependent phosphate and calcium homeostasis [1,5]. It was shown that the transmembrane form of Kl is a co-receptor for FGF-receptor 1, which converts that receptor into a specific receptor for FGF23 [6,7].
The anti-aging properties of Kl might be caused by inhibition of insulin and IGF-1 signaling, since mice lacking Kl are hypoglycemic and hypersensitive to insulin [8]. This would be in line with numerous findings showing the involvement of insulin/IGF-1 signaling pathways in longevity [for review see 3]. Kl overexpressing mice have a higher resistance to oxidative stress [9]. This resistance might be induced via Kl-mediated downregulation of insulin/IGF-1 signaling, which is involved in the inactivation of FOXO transcription factors. Target genes of FOXO transcription factors are, among others, antioxidant enzymes [for review see 3].
Type-I transmembrane proteins often are shedded by metalloproteases of the ADAM (a disintegrin and metalloprotease) family. Release of ectodomains by ADAMs can have important physiological functions, for example in the case of TNFα or Notch signaling or the release of soluble Amyloid Precursor Protein (APP) by ADAM10 and ADAM17 [for review see 10,11]. Shedding can also be mediated by β-secretase, also called BACE1 (β-APP cleaving enzyme 1), a key enzyme in the pathology of Alzheimer's Disease [for review see 12]. The remaining membrane-bound fragments “stubs” are in many instances substrate for a process called regulated intramembrane proteolysis. This process is mediated by an enzymatic complex called γ-secretase [for review see 13]. BACE1 and γ-secretase are potential drug targets for the treatment of Alzheimer's Disease and the latter also for a number of Notch-driven cancer types. It is therefore important to carefully study potential substrates of these enzymes and to be aware of potential side effects caused by interfering with their function.
We here show that Kl is processed by ADAM10 and 17 and by BACE1. The remaining stubs are further processed by γ-secretase.
2. Material and Methods
Antibodies and cell lines
The following antibodies were used. Anti-flag was purchased from Sigma-Aldrich; monoclonal anti-GFP from Invitrogen; monoclonal anti-Kl (KM2119 from Kyowa Hakko Kogyo Co [14]); polyclonal anti-ADAM10 and anti-BACE1 from Abcam. HEK293 cells stably expressing PS1 and PS1(D385N), respectively, were described before [15]. MEF devoid of PS1/2 [16], ADAM10, ADAM17, ADAM10/17 and BACE1 [17,18] were described before. 293Kl (HEK293 cells stably expressing flag-tagged Kl) were described [6]. Cells were grown in DMEM supplemented with 10% FCS and Pen/Strep.
cDNA-constructs and transfections
Flag-tagged Kl was described before [6]. Human cDNA clones containing the ADAM10 or BACE1 coding region (OpenBiosystems) were cloned into pcDNA3.1(+) expression vector (Invitrogen). 293Kl and MEF cells were transfected using Lipofectamine 2000 (Invitrogen) and TurboFect (Fermentas), respectively.
Immunoprecipitation, immunoblotting and shedding assay
For shedding assays cells were plated in 6-well plates and grown to 70-80% confluency. 24h after transfection with Kl-flag (in case of MEF cells) or 48h after transfection of siRNA or 16h after drug treatment (in case of HEK293Kl), cells were washed in PBS and incubated in DMEM without serum for 3-6h. The cleared conditioned media were subjected to immunoblot analysis for Klotho protein directly or after trichloric acid precipitation. Cells were lysed in STEN-lysis buffer (50 mM Tris pH 7.6, 150 mM NaCl, 2 mM EDTA, 1% NP40 and protease inhibitor mix). Proteins were separated on 6% SDS-PAGE (for shedding experiments) or 10-20% Tris-Tricine gels (Invitrogen, for γ-secretase processing), and transferred to PVDF membranes. Membranes were cut at appropriate positions and blotted with antibodies as indicated. For quantitation, a Fuji LAS4000 Luminescent Image Analyzer was used. The ratio of cellular vs secreted Kl indicated shedding efficiency.
Drug treatments
To inhibit γ-secretase, cells were incubated overnight with 1 μM DAPT (Axxora) or 5 μM L385,345 (Sigma-Aldrich), respectively. To stimulate or inhibit ADAM mediated shedding, Phorbol-12-myristate-13-acetate (PMA) was used at 1 μM for 16h and TAPI-1 at 25 μM for 16h, respectively. To inhibit BACE1, C3 (Calbiochem) dissolved in DMSO was used at 2 μM for 16 h.
siRNA-mediated knock-down
Specific siRNAs were transfected using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol and cells and media collected 48h after transfection. For qPCR RNA was isolated with Trizol reagent (Sigma) 48h post-transfection and the PCR was performed with the SYBR Green Master Mix (Applied Biosystems, Darmstadt, Germany).
3. Results
Kl is processed by ADAM10, 17 and BACE1
Kl is shedded into extracellular fluids similar to APP, suggesting that a surface protease of the ADAM family or BACE1 might be involved. To get an initial idea if these proteases could cleave Kl, HEK293 cells stably expressing Kl (HEK293Kl) were first subjected to ADAM10 analysis (Fig. 1A). Activation of ADAMs by PMA increased shedding of Kl by 50%, whereas the metalloprotease inhibitor TAPI-1 decreased shedding of Kl by 90%. More specifically, overexpression of ADAM10 increased shedding of Kl by 60% whereas knockdown by two different siRNAs decreased shedding by 25 and 30%, respectively, suggesting that ADAM10 is involved in shedding of Kl (Fig. 1A). Efficient knock-down of ADAM10 but not control siRNAs was verified on mRNA level (Fig. 1B). Next we analyzed if BACE1 can mediate shedding of Kl. Overexpression of BACE1 cDNA but not expression vector alone induced shedding of Kl (Fig. 1C). Two different BACE1 siRNAs but not control siRNA decreased BACE1-induced shedding of Kl by 70%. In addition, using a BACE1 inhibitor, C3, reduced the BACE1-induced shedding of Kl by 50% (Fig. 1B). Taken together, these data suggest that ADAM10 and BACE1 can shed Kl. Overexpression of an enzyme can only give hints to its potential role, and knock-down with siRNAs might cause off-target effects. We therefore made use of mouse embryonic fibroblasts (MEF) devoid of ADAM10 and BACE1, respectively. In addition, we tested MEF cells deficient in ADAM17 and deficient in both ADAM10 and 17. Transient transfection of Kl in these MEF cells showed a strong reduction in shedding in all KO cells, with some variability between different lines (Fig. 2A, quantitation in 2B). A very strong reduction in shedding was observed in ADAM10/17 double KO MEFs, again supporting the idea that these two proteases are involved in shedding of Kl. Taken together, these data indicate that the processing of Kl is similar to APP in that both α- and β-secretases are able to cleave Kl.
Fig. 1.
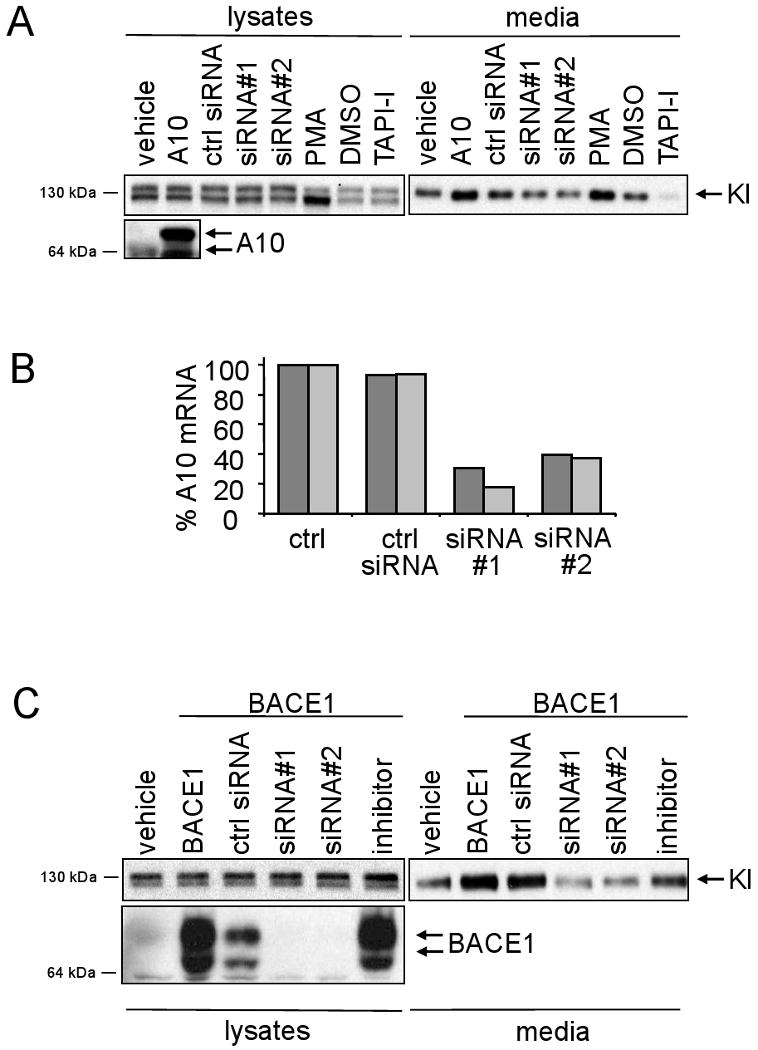
Kl is shedded by ADAM10 and BACE1. A) 293Kl cells were transiently transfected with ADAM10 (A10) or empty vector (vehicle) or with siRNAs as indicated or treated with PMA or TAPI-1 for 16h and shedding of Kl was assayed after 4h. After Western blotting, lysates and media were probed with Kl antibodies and relevant lysates with ADAM10 antibodies. B) mRNA from cells transfected with transfection reagent only (ctrl) or control siRNAs or two different siRNAs against ADAM10 was isolated and qPCR using primers specific for ADAM10 was performed. mRNA level in ctrl cells was set to 100% and levels in siRNA treated cells related to that. Results from two independent experiments are shown. C) 293Kl cells were transiently transfeced with empty vector (vehicle) or with BACE1 cDNA or with BACE1 cDNA and in addition with siRNAs as indicated or treated with BACE inhibitor. Thereafter shedding of Kl was assayed as in A). Lysates in addition were probed with BACE1 antibodies.
Fig. 2.
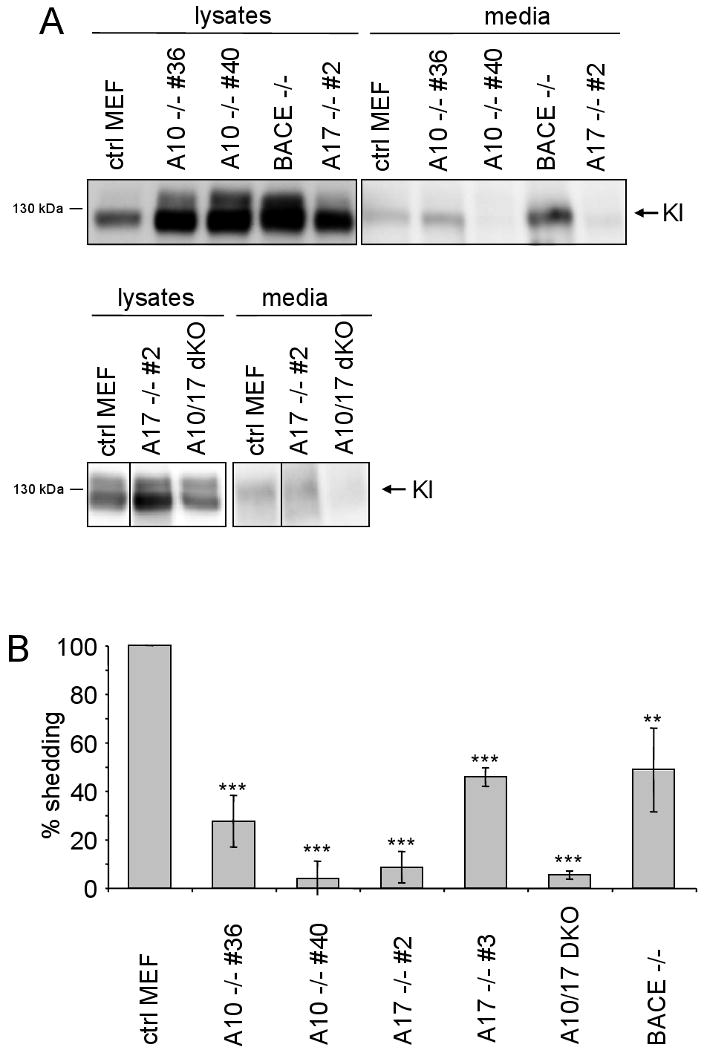
Kl is shedded by ADAM10, 17 and BACE1. A) MEFs cells derived from KO mice as indicated were transiently transfected with Kl-flag and shedding was assayed after 24h. After Western blotting, lysates and media were probed with Kl antibody. Representative gels are shown. B) Quantitation of shedding. The ratio of shedded Kl in the medium versus Kl in cell lysates was determined in all MEF lines. In ctrl MEF cells this ratio was set to 100% and the ratios from the other cell lines related to that. Error bars depict standard deviation from 4-7 independent experiments. ***, p<0.001; **, p<0.01.
Kl is processed by γ-secretase
The shedding of Kl is reminiscent of APP, Notch and many other type I transmembrane proteins. In all these cases the remaining, membrane-bound stub is substrate for γ-secretase. To test if γ-secretase would cleave the Kl stub, we incubated HEK293Kl cells with or without two different γ-secretase inhibitors, L-685,458 and DAPT (Fig. 3A). In the presence of both inhibitors a small 5 kDa fragment was observed, which was hardly seen in the absence of the inhibitor. To further test if γ-secretase is mediating the processing of the Kl-stub, we transfected Kl-flag in HEK293 cell overexpressing functional PS1(wt) or non-functional PS1(D385N) [15]. Only in PS1(D385N) cells without functional γ-secretase the 5 kDa fragment was visible, suggesting that it is a substrate of γ-secretase which is processed in PS1(wt) cells (Fig. 3B). Further evidence for γ-secretase processing of the Kl-stub was provided by transiently transfecting Kl-flag in mouse embryonic fibroblasts (MEF) derived from PS1-/-/PS2-/- double KO mice and from wild type mice, respectively. Fig. 3C demonstrated that the 5 kDa Kl-stub could be detected only in transfected MEF PS1-/-/PS2-/-, but not in transfected wild type MEF cells, strongly suggesting that the Kl-stub is substrate for γ-secretase (Fig. 3C). The specificity of the immunoprecipitation was demonstrated in Fig. 3D. Taken together, these data show that after shedding of the extracellular domain Kl is a substrate for intramembrane proteolysis by γ-secretase.
Fig. 3.
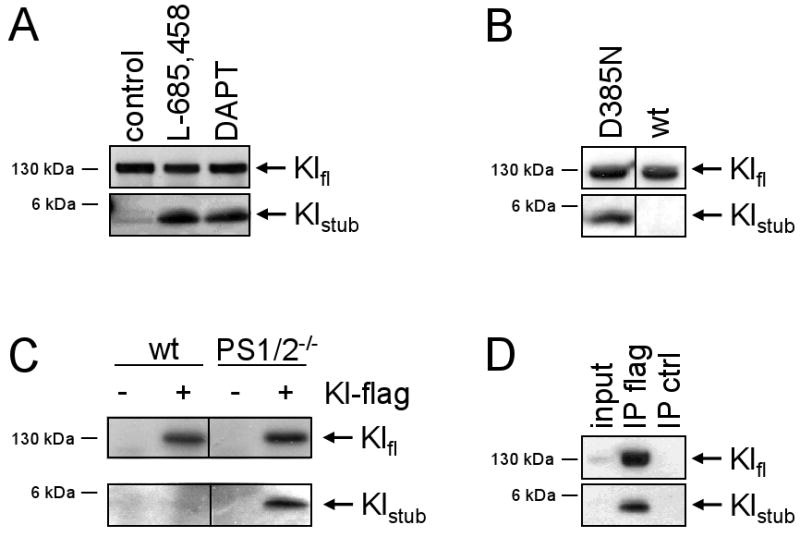
Kl is processed by γ-secretase. A) 293Kl cells were incubated with or without two different γ-secretase inhibitors for 24h as indicated, lysed and immunoprecipitated with flag-antibody, separated on 10-20% Tris-Tricine gels, blotted and probed with flag-antibody. B) Kl-flag cDNA was transiently transfected in HEK293 cells stably expressing PS1wt (wt) or dominant negative PS1(D385N). Lysates were immunoprecipitated and processed as in A). C) MEFs derived from wild type mice (wt) or PS1/2 double knock-out mice were transiently transfected with Kl-flag and processed as in A). D) To demonstrate specificity of the antibody lysates from HEK293Kl cells were subjected to immunoprecipitation with flag antibody (IP flag) and with beads only (IP ctrl) and processed as in A). In the input lane 1/25th of the lysate used for IP was loaded.
4. Discussion
Many of the physiological functions of Kl can be attributed to its membrane bound full-length form, for example its role as coreceptor for FGF23 [6,7]. Its limited expression mainly in the kidney and choroid plexus together with the occurrence of the secreted form of Kl in serum and CSF as well as the plethora of affected tissues in Kl deficient mice suggest a hormone like role of the secreted Kl [for review see 3]. One function of the secreted Kl is to antagonize wnt signaling [19], another the activation of the calcium channel TRPV5 [20]. It is therefore important to dissect in molecular detail Kl processing and its regulation that would influence the ratio of full length versus secreted form. Here we show that Kl processing is similar to APP, Notch and other cell surface proteins. Kl can be shedded by ADAM10 and 17. Our data confirm earlier findings by Chen et al [21] and extend their findings by showing that also BACE1 is able to shed Kl. The findings presented here suggest future experiments to study the role of BACE1 in Kl processing in vivo, since BACE1 inhibitors developed to reduce amyloid-beta load in Alzheimer's Disease patients might interfere with Kl-mediated physiological processes. BACE KO mice do not have obvious age-related phenotypes, but such phenotypes might have been overlooked because the potential connection to Kl was not known. Using inhibitors, dominant-negative PS1 mutations as well as PS1-/-/PS2-/- cells we demonstrate that the Kl-stub remaining after shedding is substrate for γ-secretase. γ-secretase cleavage might serve to remove the stub from the membrane, as has been suggested for other substrates [22]. It remains to be shown whether the intracellular domain, which would be very small, has a physiological function. Efforts to visualize and purify this peptide were unsuccessful so far, probably because of its very low half-life (DR and CK, unpublished). The work presented here provides the basis for future analysis of the role of shedding in the physiology of Kl. It will be interesting to investigate if the ratio full-length/secreted form is changed during aging or under pathophysiological conditions and what the underlying mechanisms are.
Acknowledgments
This work was supported by a grant from the IZKF Jena (to CK), by the Deutsche Forschungsgemeinschaft (SFB415, to PS and KR), Genzyme (GRIP, to MK) and NIH (R01AG19712 to MK). We thank Sven Lammich (Adolf-Butenandt-Institut, München, Germany) for providing cell lines.
List of abbreviations
- ADAM
a disintegrin and metalloprotease
- APP
amyloid precursor protein
- BACE1
β-APP cleaving enzyme 1
- Kl
Klotho
- MEF
mouse embryonic fibroblast
- PS1
Presenilin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008 doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 4.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 5.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 6.Kurosu H, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 8.Utsugi T, et al. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49:1118–23. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–34. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deuss M, Reiss K, Hartmann D. Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res. 2008;5:187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- 11.Reiss K, Saftig P. The “A Disintegrin And Metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–37. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Hunt CE, Turner AJ. Cell biology, regulation and inhibition of beta-secretase (BACE-1) Febs J. 2009;276:1845–59. doi: 10.1111/j.1742-4658.2009.06929.x. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy JV, Twomey C, Wujek P. Presenilin-dependent regulated intramembrane proteolysis and gamma-secretase activity. Cell Mol Life Sci. 2009 doi: 10.1007/s00018-009-8435-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato Y, et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597–602. doi: 10.1006/bbrc.1999.2009. [DOI] [PubMed] [Google Scholar]
- 15.Lammich S, et al. Presenilin-dependent Intramembrane Proteolysis of CD44 Leads to the Liberation of Its Intracellular Domain and the Secretion of an Abeta - like Peptide. J Biol Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 16.Herreman A, Van Gassen G, Bentahir M, Nyabi O, Craessaerts K, Mueller U, Annaert W, De Strooper B. gamma-Secretase activity requires the presenilin-dependent trafficking of nicastrin through the Golgi apparatus but not its complex glycosylation. J Cell Sci. 2003;116:1127–36. doi: 10.1242/jcs.00292. [DOI] [PubMed] [Google Scholar]
- 17.Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. Embo J. 2005;24:742–52. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gall SM, et al. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha, L-selectin, and tumor necrosis factor alpha. Mol Biol Cell. 2009;20:1785–94. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 20.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 21.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]


