Figure 3.
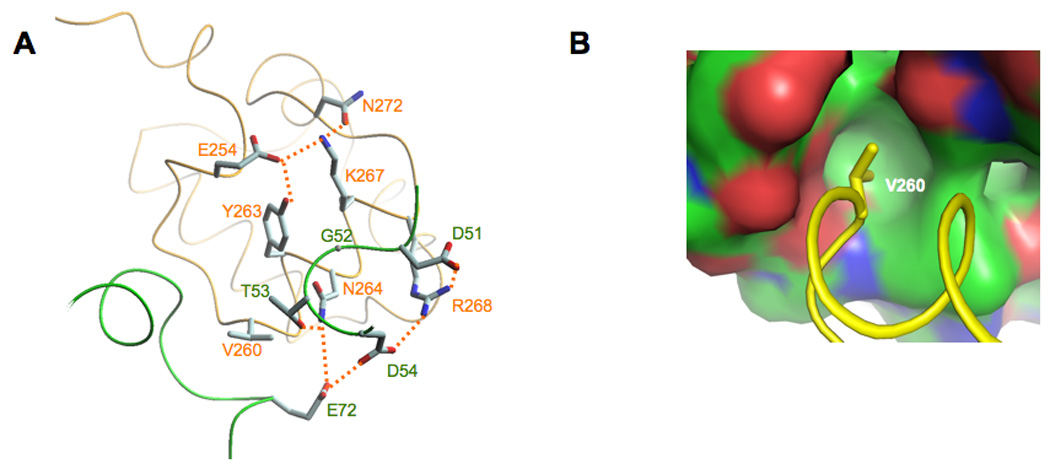
Molecular details of the Spx/αCTD interface. A. In both the in vivo- and the in vitro-assembled structures, αCTD residue Asn264 and Arg268 interact with Spx residues Asp51, Thr53 and Asp54. The residues that contribute to the overall Spx/αCTD interface are highlighted (hydrogen bonds are shown as dashed lines). B. αCTD residue Val260 projects into a hydrophobic pocket in Spx (the surface is colored in green for carbon atoms, red for oxygens and blue for nitrogens).
