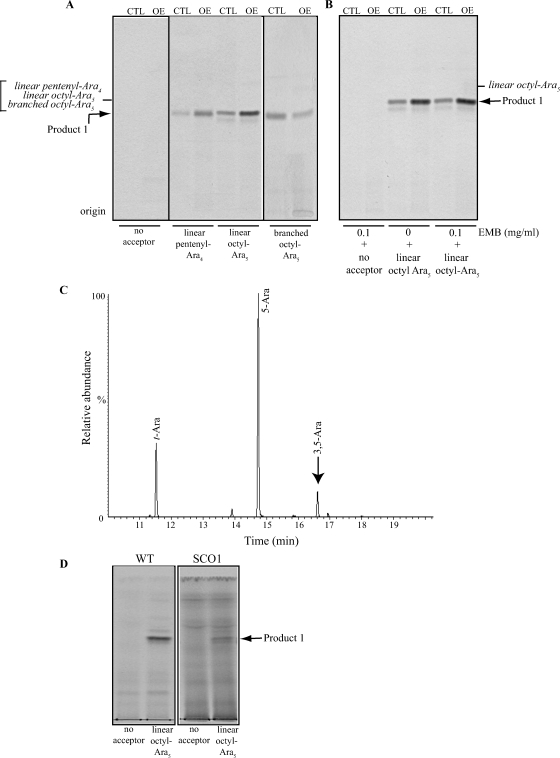
Fig. 6.
Arabinofuranosyltransferase assays using synthetic arabinosyl acceptors. (A) TLC autoradiographs of the products of the reactions using mc2155/pJAM2 (CTL) and mc2155/pJAMRv0236c (OE) cell-free extracts as enzyme sources, p[14C]Rpp as the donor substrate and different synthetic arabinofuranosyl acceptors. The lower minor product formed in the reaction utilizing a linear Ara5 acceptor is likely to result from the activity of a β-1,2 AraT, consistent with previous studies using mycobacterial extracts and short linear Araf acceptors (Birch et al. 2008). The synthesis of this product is not affected by the overexpression of aftD. (B) The same assays using linear Ara5 acceptor were run in the presence of 0.1 mg/mL ethambutol (EMB). Samples were prepared and analyzed as described under Material and methods. Twenty percent of each reaction was loaded onto the TLC plate. The products of the reactions were identified by co-migration with synthetic arabinofuranosyl standards (italicized). The presence of different aglycon moieties (pentenyl or octyl) on the synthetic acceptors accounts for the similar Rf of the radiolabeled Ara5 and Ara6 products on the TLC plate. (C) GC/MS analysis of the Ara6 product of the reaction. The per-O-methylated purified product was hydrolyzed with 2 M TFA, reduced, per-O-acetylated, and analyzed as described under Material and methods. (D) Assays using WT mc2155 (WT) and SCO1 cell-free extracts, p[14C]Rpp as the donor substrate and linear synthetic Ara5 as the acceptor substrate.